|
Tyramine
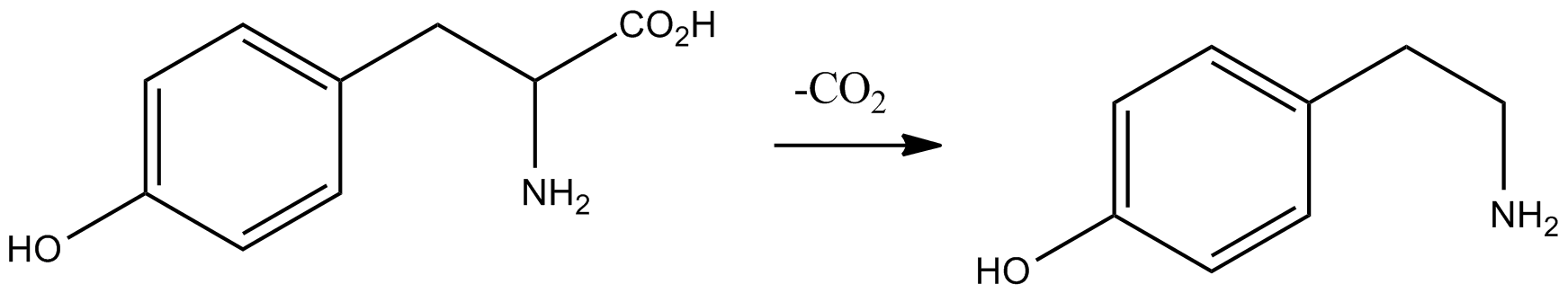
Tyramine ( ) (also spelled tyramin), also known under several other names, is a naturally occurring trace amine derived from the amino acid tyrosine. Tyramine acts as a catecholamine releasing agent. Notably, it is unable to cross the blood-brain barrier, resulting in only non-psychoactive peripheral sympathomimetic effects following ingestion. A hypertensive crisis can result, however, from ingestion of tyramine-rich foods in conjunction with the use of monoamine oxidase inhibitors (MAOIs). Occurrence Tyramine occurs widely in plants and animals, and is metabolized by various enzymes, including monoamine oxidases. In foods, it often is produced by the decarboxylation of tyrosine during fermentation or decay. Foods that are fermented, cured, pickled, aged, or spoiled have high amounts of tyramine. Tyramine levels go up when foods are at room temperature or go past their freshness date. Specific foods containing considerable amounts of tyramine include: * strong or ag ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
N-Methyltyramine
''N''-Methyltyramine (NMT), also known as 4-hydroxy-''N''-methylphenethylamine, is a human trace amine and natural substituted phenethylamine, phenethylamine alkaloid found in a variety of plants.T. A. Smith (1977). "Phenethylamine and related compounds in plants." ''Phytochemistry'' 16 9 – 18. As the name implies, it is the N-methyl analog of tyramine, which is a well-known biogenic amine, biogenic trace amine with which NMT shares many pharmacological properties. Biosynthetically, NMT is produced by the N-methylation of tyramine via the action of the enzyme phenylethanolamine N-methyltransferase, phenylethanolamine ''N''-methyltransferase in humans and tyramine N-methyltransferase, tyramine ''N''-methyltransferase in plants. Occurrence N-methyltyramine seems to be quite widely distributed in plants. NMT was isolated as a natural product for the first time, from germinating barley roots, by Kirkwood and Marion in 1950. These chemists found that 600 g of barley, after germinat ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Octopamine
Octopamine (molecular formula C8H11NO2; also known as OA, and also norsynephrine, ''para''-octopamine and others) is an organic chemical closely related to norepinephrine, and synthesized biologically by a homologous pathway. Octopamine is often considered the major "fight-or-flight" neurohormone of invertebrates. Its name is derived from the fact that it was first identified in the salivary glands of the octopus. In many types of invertebrates octopamine is an important neurotransmitter and hormone. In protostomes — arthropods, molluscs, and several types of worms — it substitutes for norephinephrine and performs functions apparently similar to those of norepinephrine in mammals, functions that have been described as mobilizing the body and nervous system for action. In mammals octopamine is found only in trace amounts, and no biological function has been solidly established for it. It is also found naturally in numerous plants, including bitter orange. Octopamine has bee ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Monoamine Oxidase Inhibitor
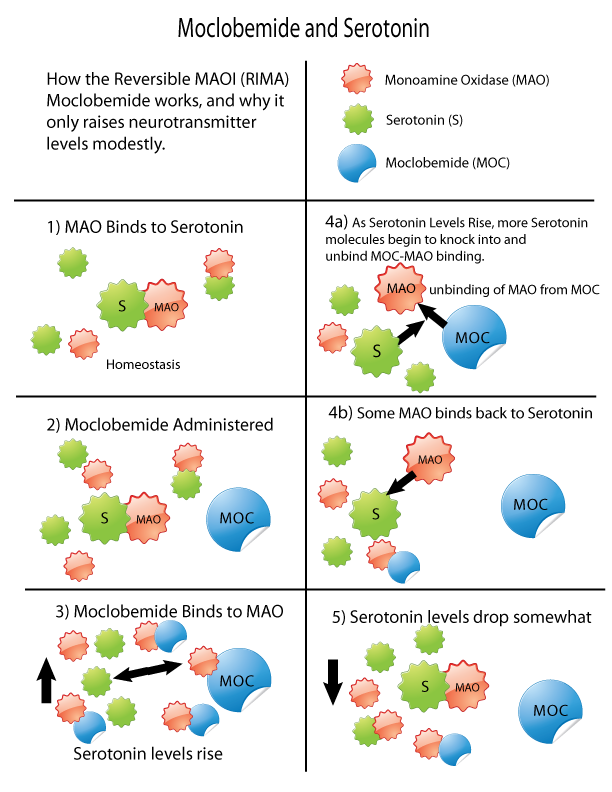
Monoamine oxidase inhibitors (MAOIs) are a class of drugs that inhibit the activity of one or both monoamine oxidase enzymes: monoamine oxidase A (MAO-A) and monoamine oxidase B (MAO-B). They are best known as effective antidepressants, especially for treatment-resistant depression and atypical depression. They are also used to treat panic disorder, social anxiety disorder, Parkinson's disease, and several other disorders. Reversible inhibitors of monoamine oxidase A (RIMAs) are a subclass of MAOIs that selectively and reversibly inhibit the MAO-A enzyme. RIMAs are used clinically in the treatment of depression and dysthymia. Due to their reversibility, they are safer in single-drug overdose than the older, irreversible MAOIs, and weaker in increasing the monoamines important in depressive disorder. RIMAs have not gained widespread market share in the United States. Medical uses MAOIs have been found to be effective in the treatment of panic disorder with agoraphobia ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
CYP2D6
Cytochrome P450 2D6 (CYP2D6) is an enzyme that in humans is encoded by the ''CYP2D6'' gene. ''CYP2D6'' is primarily expressed in the liver. It is also highly expressed in areas of the central nervous system, including the substantia nigra. CYP2D6, a member of the cytochrome P450 mixed-function oxidase system, is one of the most important enzymes involved in the metabolism of xenobiotics in the body. In particular, CYP2D6 is responsible for the metabolism and elimination of approximately 25% of clinically used drugs, via the addition or removal of certain functional groups – specifically, hydroxylation, demethylation, and dealkylation. CYP2D6 also activates some prodrugs. This enzyme also metabolizes several endogenous substances, such as hydroxytryptamines, neurosteroids, and both ''m''-tyramine and ''p''-tyramine which CYP2D6 metabolizes into dopamine in the brain and liver. Considerable variation exists in the efficiency and amount of CYP2D6 enzyme produced betwee ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Dopamine
Dopamine (DA, a contraction of 3,4-dihydroxyphenethylamine) is a neuromodulatory molecule that plays several important roles in cells. It is an organic compound, organic chemical of the catecholamine and phenethylamine families. Dopamine constitutes about 80% of the catecholamine content in the brain. It is an amine synthesized by removing a carboxyl group from a molecule of its precursor (chemistry), precursor chemical, L-DOPA, which is biosynthesis, synthesized in the brain and kidneys. Dopamine is also synthesized in plants and most animals. In the brain, dopamine functions as a neurotransmitter—a chemical released by neurons (nerve cells) to send signals to other nerve cells. Neurotransmitters are synthesized in specific regions of the brain, but affect many regions systemically. The brain includes several distinct dopaminergic pathway, dopamine pathways, one of which plays a major role in the motivational component of reward system, reward-motivated behavior. The anticipa ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Trace Amine
Trace amines are an endogenous group of trace amine-associated receptor 1 (TAAR1) agonists – and hence, monoaminergic neuromodulators – that are structurally and metabolically related to classical monoamine neurotransmitters. Compared to the classical monoamines, they are present in trace concentrations. They are distributed heterogeneously throughout the mammalian brain and peripheral nervous tissues and exhibit high rates of metabolism. Although they can be synthesized within parent monoamine neurotransmitter systems, there is evidence that suggests that some of them may comprise their own independent neurotransmitter systems. Trace amines play significant roles in regulating the quantity of monoamine neurotransmitters in the synaptic cleft of monoamine neurons with . They have well-characterized presynaptic ''amphetamine-like'' effects on these monoamine neurons via TAAR1 activation; specifically, by activating TAAR1 in neurons they promote the release and prevent reuptak ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Monoamine Oxidase B
Monoamine oxidase B, also known as MAOB, is an enzyme that in humans is encoded by the ''MAOB'' gene. The protein encoded by this gene belongs to the flavin monoamine oxidase family. It is an enzyme located in the outer mitochondrial membrane. It catalyzes the oxidative deamination of biogenic and xenobiotic amines and plays an important role in the catabolism of neuroactive and vasoactive amines in the central nervous system and peripheral tissues (such as dopamine). This protein preferentially degrades benzylamine and phenethylamine. Similarly to monoamine oxidase A (MAOA), it also degrades dopamine hough some new research contradicts this, suggesting that MAOB does ''not'' directly degrade dopamine, but is responsible for GABA synthesis Structure Monoamine oxidase B has a hydrophobic bipartite elongated cavity that (for the "open" conformation) occupies a combined volume close to 700 Å3. hMAO-A has a single cavity that exhibits a rounder shape and is larger in volume t ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Dopamine Beta-hydroxylase
Dopamine beta-hydroxylase (DBH), also known as dopamine beta-monooxygenase, is an enzyme () that in humans is encoded by the DBH gene. Dopamine beta-hydroxylase catalyzes the conversion of dopamine to norepinephrine. The three substrates of the enzyme are dopamine, vitamin C (ascorbate), and O2. The products are norepinephrine, dehydroascorbate, and H2O. DBH is a 290 kDa copper-containing oxygenase consisting of four identical subunits, and its activity requires ascorbate as a cofactor. It is the only enzyme involved in the synthesis of small-molecule neurotransmitters that is membrane-bound, making norepinephrine the only known transmitter synthesized inside vesicles. It is expressed in noradrenergic neurons of the central nervous system (i.e. locus coeruleus) and peripheral nervous systems (i.e. sympathetic ganglia), as well as in chromaffin cells of the adrenal medulla. Mechanism of catalysis Based on the observations of what happens when there is no substrate, o ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Monoamine Oxidase
Monoamine oxidases (MAO) () are a family of enzymes that catalyze the oxidation of monoamines, employing oxygen to clip off their amine group. They are found bound to the outer membrane of mitochondria in most cell types of the body. The first such enzyme was discovered in 1928 by Mary Bernheim in the liver and was named tyramine oxidase. The MAOs belong to the protein family of flavin-containing amine oxidoreductases. MAOs are important in the breakdown of monoamines ingested in food, and also serve to inactivate monoamine neurotransmitters. Because of the latter, they are involved in a number of psychiatric and neurological diseases, some of which can be treated with monoamine oxidase inhibitors (MAOIs) which block the action of MAOs. Subtypes and tissue distribution In humans there are two types of MAO: MAO-A and MAO-B. * Both are found in neurons and astroglia. * Outside the central nervous system: ** MAO-A is also found in the liver, pulmonary vascular endothelium, gas ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Phenylethanolamine N-methyltransferase
Phenylethanolamine ''N''-methyltransferase (PNMT) is an enzyme found primarily in the adrenal medulla that converts norepinephrine (noradrenaline) to epinephrine (adrenaline). It is also expressed in small groups of neurons in the human brain and in selected populations of cardiomyocytes. Structure PNMT is a protein whose encoding gene is found on chromosome 17 in humans. It consists of 4 exons and is a 30 kDa protein. It shares many properties found among the other methyltransferases. It is closest in sequence to glycine-''N''-methyl transferase ( GNMT). It also shares many structural properties like the shape of the folding lip with catechol-O-methyl transferase (COMT), though it shares less sequence identity. Several features of the structure like this folding lip suggest that PNMT is a recent adaptation to the catecholamine synthesizing enzyme family, evolving later than COMT, but before other methyltransferases like GNMT. ''S''-adenosyl-L-methionine (SAM) is a requir ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Flavin-containing Monooxygenase 3
Flavin-containing monooxygenase 3 (FMO3), also known as dimethylaniline monooxygenase -oxide-forming3 and trimethylamine monooxygenase, is a flavoprotein enzyme () that in humans is encoded by the ''FMO3'' gene. This enzyme catalyzes the following chemical reaction, among others: :trimethylamine + NADPH + H+ + O2 \rightleftharpoons trimethylamine ''N''-oxide + NADP+ + H2O FMO3 is the main flavin-containing monooxygenase isoenzyme that is expressed in the liver of adult humans. The human FMO3 enzyme catalyzes several types of reactions, including: the of primary, secondary, and tertiary amines; the of nucleophilic sulfur-containing compounds; and the of the anti-cancer agent dimethylxanthenone acetic acid (DMXAA). FMO3 is the primary enzyme in humans which catalyzes the ''N''-oxidation of trimethylamine into trimethylamine ''N''-oxide; FMO1 also does this, but to a much lesser extent than FMO3. Genetic deficiencies of the FMO3 enzyme cause primary trimethylaminuria, also k ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
4-Hydroxyphenylacetaldehyde
4-Hydroxyphenylacetaldehyde, also known as ''p''-hydroxyphenylacetaldehyde, is a natural product with the formula HOC6H4CH2CHO. It is a derivative of phenylacetaldehyde and occurs as a white solid at room temperature. Synthesis 4-Hydroxyphenylacetaldehyde can be synthesized from a parsley tyrosine -Tyrosine or tyrosine (symbol Tyr or Y) or 4-hydroxyphenylalanine is one of the 20 standard amino acids that are used by cells to synthesize proteins. It is a non-essential amino acid with a polar side group. The word "tyrosine" is from the Gr ... decarboxylase (L-Tyrosine, L-tyrosine). Occurrence 4-Hydroxyphenylacetaldehyde is produced from the metabolism of tyramine by monoamine oxidase (MAO) enzymes in humans and the primary amine oxidase, tyramine oxidase (tynA) enzyme in ''Escherichia coli''. In both species, it is subsequently metabolized into 4-hydroxyphenylacetate by aldehyde dehydrogenase (ALDH) enzymes in humans and the phenylacetaldehyde dehydrogenase (feaB) enzyme in ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |