|
Membrane Trafficking
Membrane vesicle trafficking in eukaryotic animal cells involves movement of biochemical signal molecules from synthesis-and-packaging locations in the Golgi body to specific release locations on the inside of the plasma membrane of the secretory cell. It takes place in the form of Golgi membrane-bound micro-sized vesicles, termed membrane vesicles (MVs). In this process, the packed cellular products are released or secreted outside the cell, across its plasma membrane. On the other hand, the vesicular membrane is retained and recycled by the secretory cells. This phenomenon has a major role in synaptic neurotransmission, endocrine secretion, mucous secretion, granular-product secretion by neutrophils, and other phenomena. The scientists behind this discovery were awarded Nobel prize for the year 2013. In prokaryotic, gram-negative bacterial cells, membrane vesicle trafficking is mediated through bacterial outer membrane bounded nano-sized vesicles, called bacterial outer m ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Vesicle (biology And Chemistry)
In cell biology, a vesicle is a structure within or outside a cell, consisting of liquid or cytoplasm enclosed by a lipid bilayer. Vesicles form naturally during the processes of secretion ( exocytosis), uptake ( endocytosis) and transport of materials within the plasma membrane. Alternatively, they may be prepared artificially, in which case they are called liposomes (not to be confused with lysosomes). If there is only one phospholipid bilayer, the vesicles are called '' unilamellar liposomes''; otherwise they are called ''multilamellar liposomes''. The membrane enclosing the vesicle is also a lamellar phase, similar to that of the plasma membrane, and intracellular vesicles can fuse with the plasma membrane to release their contents outside the cell. Vesicles can also fuse with other organelles within the cell. A vesicle released from the cell is known as an extracellular vesicle. Vesicles perform a variety of functions. Because it is separated from the cytosol, the ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Exocytosis
Exocytosis () is a form of active transport and bulk transport in which a cell transports molecules (e.g., neurotransmitters and proteins) out of the cell ('' exo-'' + '' cytosis''). As an active transport mechanism, exocytosis requires the use of energy to transport material. Exocytosis and its counterpart, endocytosis, are used by all cells because most chemical substances important to them are large polar molecules that cannot pass through the hydrophobic portion of the cell membrane by passive means. Exocytosis is the process by which a large amount of molecules are released; thus it is a form of bulk transport. Exocytosis occurs via secretory portals at the cell plasma membrane called porosomes. Porosomes are permanent cup-shaped lipoprotein structure at the cell plasma membrane, where secretory vesicles transiently dock and fuse to release intra-vesicular contents from the cell. In exocytosis, membrane-bound secretory vesicles are carried to the cell membrane, where ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Microtubule
Microtubules are polymers of tubulin that form part of the cytoskeleton and provide structure and shape to eukaryotic cells. Microtubules can be as long as 50 micrometres, as wide as 23 to 27 nm and have an inner diameter between 11 and 15 nm. They are formed by the polymerization of a dimer of two globular proteins, alpha and beta tubulin into protofilaments that can then associate laterally to form a hollow tube, the microtubule. The most common form of a microtubule consists of 13 protofilaments in the tubular arrangement. Microtubules play an important role in a number of cellular processes. They are involved in maintaining the structure of the cell and, together with microfilaments and intermediate filaments, they form the cytoskeleton. They also make up the internal structure of cilia and flagella. They provide platforms for intracellular transport and are involved in a variety of cellular processes, including the movement of secretory vesicles, ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
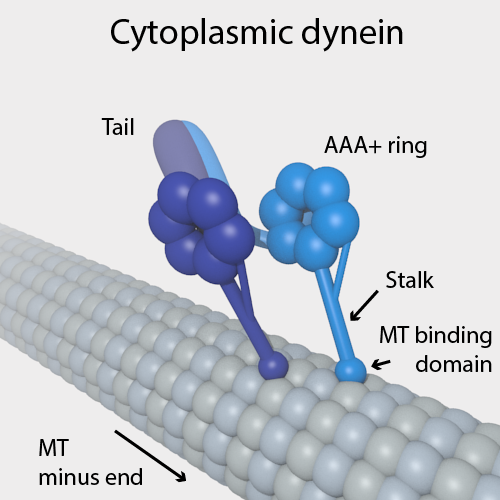
Dynein
Dyneins are a family of cytoskeletal motor proteins that move along microtubules in cells. They convert the chemical energy stored in ATP to mechanical work. Dynein transports various cellular cargos, provides forces and displacements important in mitosis, and drives the beat of eukaryotic cilia and flagella. All of these functions rely on dynein's ability to move towards the minus-end of the microtubules, known as retrograde transport; thus, they are called "minus-end directed motors". In contrast, most kinesin motor proteins move toward the microtubules' plus-end, in what is called anterograde transport. Classification Dyneins can be divided into two groups: cytoplasmic dyneins and axonemal dyneins, which are also called ciliary or flagellar dyneins. * cytoplasmic ** heavy chain: DYNC1H1, DYNC2H1 ** intermediate chain: DYNC1I1, DYNC1I2 ** light intermediate chain: DYNC1LI1, DYNC1LI2, DYNC2LI1 ** light chain: DYNLL1, DYNLL2, DYNLRB1, DYNLRB2, DYNLT1, DYN ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Kinesin
A kinesin is a protein belonging to a class of motor proteins found in eukaryotic cells. Kinesins move along microtubule (MT) filaments and are powered by the hydrolysis of adenosine triphosphate (ATP) (thus kinesins are ATPases, a type of enzyme). The active movement of kinesins supports several cellular functions including mitosis, meiosis and transport of cellular cargo, such as in axonal transport, and intraflagellar transport. Most kinesins walk towards the plus end of a microtubule, which, in most cells, entails transporting cargo such as protein and membrane components from the center of the cell towards the periphery. This form of transport is known as anterograde transport. In contrast, dyneins are motor proteins that move toward the minus end of a microtubule in retrograde transport. Discovery Kinesins were discovered in 1985, based on their motility in cytoplasm extruded from the giant axon of the squid. They turned out as MT-based anterograde intracellular tra ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Myosin
Myosins () are a superfamily of motor proteins best known for their roles in muscle contraction and in a wide range of other motility processes in eukaryotes. They are ATP-dependent and responsible for actin-based motility. The first myosin (M2) to be discovered was in 1864 by Wilhelm Kühne. Kühne had extracted a viscous protein from skeletal muscle that he held responsible for keeping the tension state in muscle. He called this protein ''myosin''. The term has been extended to include a group of similar ATPases found in the cells of both striated muscle tissue and smooth muscle tissue. Following the discovery in 1973 of enzymes with myosin-like function in ''Acanthamoeba castellanii'', a global range of divergent myosin genes have been discovered throughout the realm of eukaryotes. Although myosin was originally thought to be restricted to muscle cells (hence '' myo-''(s) + '' -in''), there is no single "myosin"; rather it is a very large superfamily of genes whose prote ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
COPII
The Coat Protein Complex II, or COPII, is a group of proteins that facilitate the formation of vesicles to transport proteins from the endoplasmic reticulum to the Golgi apparatus or endoplasmic-reticulum–Golgi intermediate compartment. This process is termed anterograde transport, in contrast to the retrograde transport associated with the COPI complex. COPII is assembled in two parts: first an inner layer of Sar1, Sec23, and Sec24 forms; then the inner coat is surrounded by an outer lattice of Sec13 and Sec31. Function The COPII coat is responsible for the formation of vesicles from the endoplasmic reticulum (ER). These vesicles transport cargo proteins to the Golgi apparatus (in yeast) or the endoplasmic-reticulum-Golgi intermediate compartment (ERGIC, in mammals). Coat assembly is initiated when the cytosolic Ras GTPase Sar1 is activated by its guanine nucleotide exchange factor Sec12. Activated Sar1-GTP inserts itself into the ER membrane, binding preferentially to area ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
COPI
COPI is a coatomer, a protein complex that coats vesicles transporting proteins from the ''cis'' end of the Golgi complex back to the rough endoplasmic reticulum (ER), where they were originally synthesized, and between Golgi compartments. This type of transport is ''retrograde transport'', in contrast to the ''anterograde transport'' associated with the COPII protein. The name "COPI" refers to the specific coat protein complex that initiates the budding process on the ''cis''-Golgi membrane. The coat consists of large protein subcomplexes that are made of seven different protein subunits, namely α, β, β', γ, δ, ε and ζ. Coat proteins Coat protein, or COPI, is an ADP ribosylation factor (ARF)-dependent protein involved in membrane traffic. COPI was first identified in retrograde traffic from the ''cis''-Golgi to the rough endoplasmic reticulum (ER) and is the most extensively studied of ARF-dependent adaptors. COPI consists of seven subunits which compose the hetero ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Clathrin
Clathrin is a protein that plays a major role in the formation of coated vesicles. Clathrin was first isolated and named by Barbara Pearse in 1976. It forms a triskelion shape composed of three clathrin heavy chains and three light chains. When the triskelia interact they form a polyhedral lattice that surrounds the vesicle, hence the protein's name, which is derived from the Latin ''clathrum'' meaning lattice. Coat-proteins, like clathrin, are used to build small vesicles in order to transport molecules within cells. The endocytosis and exocytosis of vesicles allows cells to communicate, to transfer nutrients, to import signaling receptors, to mediate an immune response after sampling the extracellular world, and to clean up the cell debris left by tissue inflammation. The endocytic pathway can be hijacked by viruses and other pathogens in order to gain entry to the cell during infection. Structure The clathrin triskelion is composed of three clathrin heavy chains inte ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Coatomer
The coatomer is a protein complex that coats membrane-bound transport vesicles. Two types of coatomers are known: *COPI (retrograde transport from trans-Golgi network to cis-Golgi network and endoplasmic reticulum) *COPII (anterograde transport from ER to the cis-Golgi) Coatomers are functionally analogous and evolutionarily homologous to clathrin adaptor proteins, also known as adaptins, which regulate endocytosis from the plasma membrane and transport from the trans-Golgi network to lysosomes. Structure The coatomer protein complex is made up of seven nonidentical protein subunits. These seven nonidentical protein subunits are part of two protein subcomplexes. The first subcomplex consists of Ret1(α-COP), Sec27(β’-COP), and Sec 28(ε-COP). The second subcomplex consists of Sec26 (β-COP), Sec21 (γ-COP), Ret2(δ-COP), and Ret3 (ζ-COP). COP I COPI is a coatomer that coats the vesicles transporting proteins from the Golgi complex to the ER. This pathway is referred t ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Lipid Bilayer Fusion
Lipids are a broad group of naturally-occurring molecules which includes fats, waxes, sterols, fat-soluble vitamins (such as vitamins A, D, E and K), monoglycerides, diglycerides, phospholipids, and others. The functions of lipids include storing energy, signaling, and acting as structural components of cell membranes. Lipids have applications in the cosmetic and food industries, and in nanotechnology. Lipids may be broadly defined as hydrophobic or amphiphilic small molecules; the amphiphilic nature of some lipids allows them to form structures such as vesicles, multilamellar/ unilamellar liposomes, or membranes in an aqueous environment. Biological lipids originate entirely or in part from two distinct types of biochemical subunits or "building-blocks": ketoacyl and isoprene groups. Using this approach, lipids may be divided into eight categories: fatty acyls, glycerolipids, glycerophospholipids, sphingolipids, saccharolipids, and polyketides (derived from c ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Motor Protein
Motor proteins are a class of molecular motors that can move along the cytoplasm of cells. They convert chemical energy into mechanical work by the hydrolysis of ATP. Flagellar rotation, however, is powered by a proton pump. Cellular functions Motor proteins are the driving force behind most active transport of proteins and vesicles in the cytoplasm. Kinesins and cytoplasmic dyneins play essential roles in intracellular transport such as axonal transport and in the formation of the spindle apparatus and the separation of the chromosomes during mitosis and meiosis. Axonemal dynein, found in cilia and flagella, is crucial to cell motility, for example in spermatozoa, and fluid transport, for example in trachea. The muscle protein myosin "motors" the contraction of muscle fibers in animals. Diseases associated with motor protein defects The importance of motor proteins in cells becomes evident when they fail to fulfill their function. For example, kinesin deficiencies ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |