|
Clathrin
Clathrin is a protein that plays a major role in the formation of coated vesicles. Clathrin was first isolated and named by Barbara Pearse in 1976. It forms a triskelion shape composed of three clathrin heavy chains and three light chains. When the triskelia interact they form a polyhedral lattice that surrounds the vesicle, hence the protein's name, which is derived from the Latin ''clathrum'' meaning lattice. Coat-proteins, like clathrin, are used to build small vesicles in order to transport molecules within cells. The endocytosis and exocytosis of vesicles allows cells to communicate, to transfer nutrients, to import signaling receptors, to mediate an immune response after sampling the extracellular world, and to clean up the cell debris left by tissue inflammation. The endocytic pathway can be hijacked by viruses and other pathogens in order to gain entry to the cell during infection. Structure The clathrin triskelion is composed of three clathrin heavy chains inter ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Endocytosis
Endocytosis is a cellular process in which substances are brought into the cell. The material to be internalized is surrounded by an area of cell membrane, which then buds off inside the cell to form a vesicle containing the ingested material. Endocytosis includes pinocytosis (cell drinking) and phagocytosis (cell eating). It is a form of active transport. History The term was proposed by De Duve in 1963. Phagocytosis was discovered by Élie Metchnikoff in 1882. Pathways Endocytosis pathways can be subdivided into four categories: namely, receptor-mediated endocytosis (also known as clathrin-mediated endocytosis), caveolae, pinocytosis, and phagocytosis Phagocytosis () is the process by which a cell uses its plasma membrane to engulf a large particle (≥ 0.5 μm), giving rise to an internal compartment called the phagosome. It is one type of endocytosis. A cell that performs phagocytosis is .... *Clathrin-mediated endocytosis is mediated by the production of smal ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
CLTC
Clathrin heavy chain 1 is a protein that in humans is encoded by the ''CLTC'' gene. Clathrin is a major protein component of the cytoplasmic face of intracellular organelles, called coated vesicles and coated pits. These specialized organelles are involved in the intracellular trafficking of receptors and endocytosis of a variety of macromolecules. The basic subunit of the clathrin coat is composed of three heavy chains and three light chains. Interactions CLTC has been shown to interact with PICALM and HGS. See also * Clathrin Clathrin is a protein that plays a major role in the formation of coated vesicles. Clathrin was first isolated and named by Barbara Pearse in 1976. It forms a triskelion shape composed of three clathrin heavy chains and three light chains. Whe ... References Further reading * * * * * * * * * * * * * * * * * * External links * {{gene-17-stub ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
CLTB (gene)
Clathrin, light chain B is a protein in humans that is encoded by the CLTB gene. Clathrin is a large, soluble protein composed of heavy and light chains. It functions as the main structural component of the lattice-type cytoplasmic face of coated pits and coated vesicles which entrap specific macromolecules during receptor-mediated endocytosis. This gene encodes one of two clathrin light chain proteins which are believed to function as regulatory elements A regulatory sequence is a segment of a nucleic acid molecule which is capable of increasing or decreasing the expression of specific genes within an organism. Regulation of gene expression is an essential feature of all living organisms and vi .... Alternative splicing results in multiple transcript variants. rovided by RefSeq, Jul 2008 References Further reading Genes on human chromosome 5 {{gene-5-stub ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Epsin
Epsins are a family of highly conserved membrane proteins that are important in creating membrane curvature. Epsins contribute to membrane deformations like endocytosis, and block Vesicle (biology), vesicle formation during mitosis. Structure Epsin contains various protein domains that aid in function. Starting at the N-terminus is the ENTH domain. ENTH stands for Epsin N-Terminal Homolog. The ENTH domain is approximately 150 amino acids long and is highly conserved across species. It is composed of seven Alpha helix, α-helices and an eighth helix that is not aligned with the seven helices that make up a superhelical fold. The role of the ENTH domain is to bind membrane lipids which is currently thought to aid in the invagination of the plasma membrane to form clathrin-coated vesicles. Additionally, located toward the C-terminus of the ENTH domain are two to three ubiquitin interacting motifs which aids in ubiquitin dependent recruitment. Following the ENTH domain there is not ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Adaptor Proteins, Vesicular Transport
Vesicular transport adaptor proteins are proteins involved in forming complexes that function in the trafficking of molecules from one subcellular location to another. These complexes concentrate the correct cargo molecules in vesicles that bud or extrude off of one organelle and travel to another location, where the cargo is delivered. While some of the details of how these adaptor proteins achieve their trafficking specificity has been worked out, there is still much to be learned. There are several human disorders associated with defects in components of these complexes including Alzheimer's and Parkinson's diseases. The proteins Most of the adaptor proteins are heterotetramers. In the AP complexes, there are two large proteins ( ∼100 k D) and two smaller proteins. One of the large proteins is termed β (beta), with β1 in the AP-1 complex, β2 in the AP-2 complex, and so on. The other large protein has different designations in the different complexes. In AP-1 i ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Barbara Pearse
Barbara Mary Frances Pearse FRS (born 24 March 1948, Wraysbury, Buckinghamshire, England) is a British biological scientist. She works at the Medical Research Council Laboratory of Molecular Biology in Cambridge, United Kingdom. Education Barbara Pearse attended the independent Lady Eleanor Holles School in Hampton in Greater London, and gained her undergraduate degree from University College London in 1969. Career She was appointed to the scientific staff of the MRC Laboratory of Molecular Biology in 1982. Research Pearse's main contributions lie in the structure of coated vesicles. Pearse first purified coated vesicles; she also discovered the clathrin coat molecule in 1975. Coated pits and vesicles were first seen in thin sections of tissue in the electron microscope by Thomas Roth and Keith Porter in 1964. The importance of them for the clearance of LDL from blood was discovered by R. G. Anderson, Michael S. Brown and Joseph L. Goldstein in 1976. Awards and honours She ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Vesicle (biology)
In cell biology, a vesicle is a structure within or outside a cell, consisting of liquid or cytoplasm enclosed by a lipid bilayer. Vesicles form naturally during the processes of secretion (exocytosis), uptake (endocytosis) and transport of materials within the plasma membrane. Alternatively, they may be prepared artificially, in which case they are called liposomes (not to be confused with lysosomes). If there is only one phospholipid bilayer, the vesicles are called ''unilamellar liposomes''; otherwise they are called ''multilamellar liposomes''. The membrane enclosing the vesicle is also a lamellar phase, similar to that of the plasma membrane, and intracellular vesicles can fuse with the plasma membrane to release their contents outside the cell. Vesicles can also fuse with other organelles within the cell. A vesicle released from the cell is known as an extracellular vesicle. Vesicles perform a variety of functions. Because it is separated from the cytosol, the inside of th ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Cell Membrane
The cell membrane (also known as the plasma membrane (PM) or cytoplasmic membrane, and historically referred to as the plasmalemma) is a biological membrane that separates and protects the interior of all cells from the outside environment (the extracellular space). The cell membrane consists of a lipid bilayer, made up of two layers of phospholipids with cholesterols (a lipid component) interspersed between them, maintaining appropriate membrane fluidity at various temperatures. The membrane also contains membrane proteins, including integral proteins that span the membrane and serve as membrane transporters, and peripheral proteins that loosely attach to the outer (peripheral) side of the cell membrane, acting as enzymes to facilitate interaction with the cell's environment. Glycolipids embedded in the outer lipid layer serve a similar purpose. The cell membrane controls the movement of substances in and out of cells and organelles, being selectively permeable to ions a ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
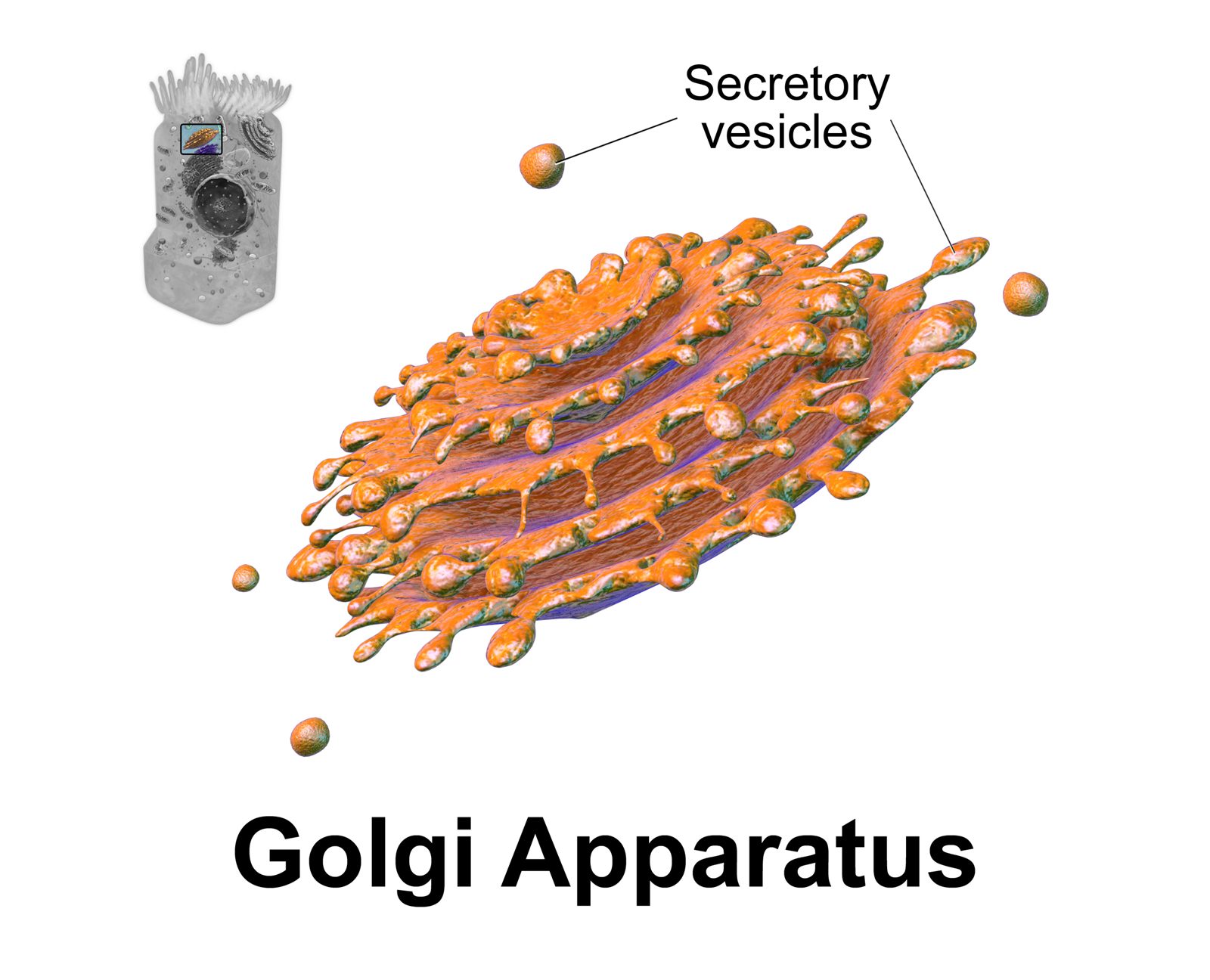
Golgi Apparatus
The Golgi apparatus (), also known as the Golgi complex, Golgi body, or simply the Golgi, is an organelle found in most eukaryotic cells. Part of the endomembrane system in the cytoplasm, it packages proteins into membrane-bound vesicles inside the cell before the vesicles are sent to their destination. It resides at the intersection of the secretory, lysosomal, and endocytic pathways. It is of particular importance in processing proteins for secretion, containing a set of glycosylation enzymes that attach various sugar monomers to proteins as the proteins move through the apparatus. It was identified in 1897 by the Italian scientist Camillo Golgi and was named after him in 1898. Discovery Owing to its large size and distinctive structure, the Golgi apparatus was one of the first organelles to be discovered and observed in detail. It was discovered in 1898 by Italian physician Camillo Golgi during an investigation of the nervous system. After first observing it under his ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Endosome
Endosomes are a collection of intracellular sorting organelles in eukaryotic cells. They are parts of endocytic membrane transport pathway originating from the trans Golgi network. Molecules or ligands internalized from the plasma membrane can follow this pathway all the way to lysosomes for degradation or can be recycled back to the cell membrane in the endocytic cycle. Molecules are also transported to endosomes from the trans Golgi network and either continue to lysosomes or recycle back to the Golgi apparatus. Endosomes can be classified as early, sorting, or late depending on their stage post internalization. Endosomes represent a major sorting compartment of the endomembrane system in cells. Function Endosomes provide an environment for material to be sorted before it reaches the degradative lysosome. For example, low-density lipoprotein (LDL) is taken into the cell by binding to the LDL receptor at the cell surface. Upon reaching early endosomes, the LDL dissociates ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Protein
Proteins are large biomolecules and macromolecules that comprise one or more long chains of amino acid residues. Proteins perform a vast array of functions within organisms, including catalysing metabolic reactions, DNA replication, responding to stimuli, providing structure to cells and organisms, and transporting molecules from one location to another. Proteins differ from one another primarily in their sequence of amino acids, which is dictated by the nucleotide sequence of their genes, and which usually results in protein folding into a specific 3D structure that determines its activity. A linear chain of amino acid residues is called a polypeptide. A protein contains at least one long polypeptide. Short polypeptides, containing less than 20–30 residues, are rarely considered to be proteins and are commonly called peptides. The individual amino acid residues are bonded together by peptide bonds and adjacent amino acid residues. The sequence of amino acid residue ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |