|
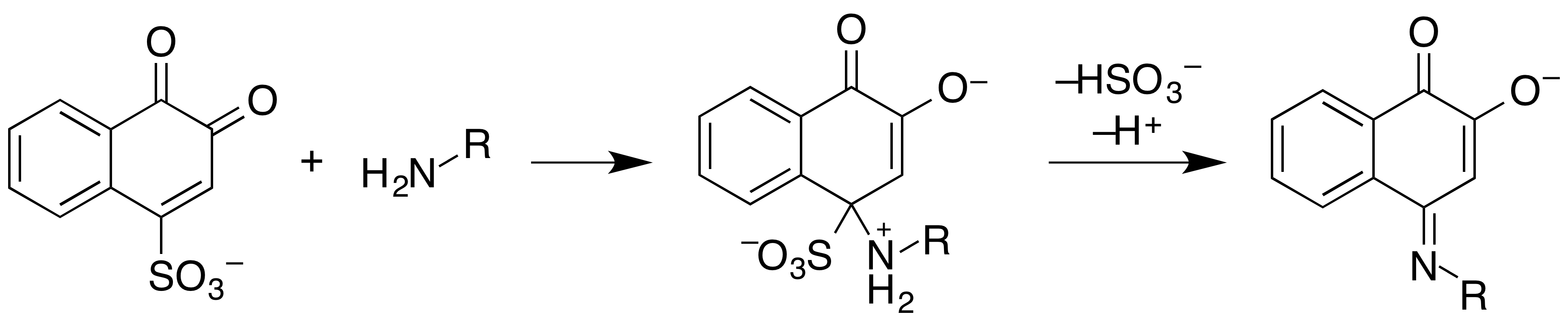
Mecke Reagent
The Mecke reagent is used as a simple spot-test to presumptively identify alkaloids as well as other compounds. It is composed of a mixture of selenous acid and concentrated sulfuric acid, which is dripped onto the substance being tested. The United States Department of Justice method for producing the reagent is the addition of 100 mL of concentrated (95–98%) sulfuric acid to 1 g of selenous acid. See also *Drug checking *Dille–Koppanyi reagent *Folin's reagent *Froehde reagent *Liebermann reagent *Mandelin reagent *Marquis reagent *Simon's reagent * Zwikker reagent References External links DHPedia - Mecke Reagent: A comprehensive list of colour reactions (inducing photographs of results) Chemical tests Analytical reagents Drug testing reagents {{chem-stub ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Alkaloids
Alkaloids are a class of basic, naturally occurring organic compounds that contain at least one nitrogen atom. This group also includes some related compounds with neutral and even weakly acidic properties. Some synthetic compounds of similar structure may also be termed alkaloids. In addition to carbon, hydrogen and nitrogen, alkaloids may also contain oxygen, sulfur and, more rarely, other elements such as chlorine, bromine, and phosphorus.Chemical Encyclopedia: alkaloids xumuk.ru Alkaloids are produced by a large variety of organisms including , , [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Para-Methoxy-N-methylamphetamine
''para''-Methoxy-''N''-methylamphetamine (also known as PMMA, Red Mitsubishi), chemically known as methyl-MA, 4-methoxy-''N''-methylamphetamine, 4-MMA) or (4-PMDA, as listed to its original physical name) is a stimulant and psychedelic drug closely related to the amphetamine-class serotonergic drug ''para''-methoxyamphetamine (PMA). PMMA is the 4-methoxy analog of methamphetamine. Little is known about the pharmacological properties, metabolism, and toxicity of PMMA; because of its structural similarity to PMA, which has known toxicity in humans, it is thought to have considerable potential to cause harmful side effects or death in overdose. In the early 2010s, a number of deaths in users of the drug MDMA were linked to misrepresented tablets and capsules of PMMA. Its effects in humans are reputedly similar to those of PMA, but slightly more empathogenic in nature. It has a reduced tendency to produce severe hyperthermia at low dosages, but at higher dosages side effects ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Chemical Tests
A chemical substance is a form of matter having constant chemical composition and characteristic properties. Some references add that chemical substance cannot be separated into its constituent elements by physical separation methods, i.e., without breaking chemical bonds. Chemical substances can be simple substances (substances consisting of a single chemical element), chemical compounds, or alloys. Chemical substances are often called 'pure' to set them apart from mixtures. A common example of a chemical substance is pure water; it has the same properties and the same ratio of hydrogen to oxygen whether it is isolated from a river or made in a laboratory. Other chemical substances commonly encountered in pure form are diamond (carbon), gold, table salt (sodium chloride) and refined sugar (sucrose). However, in practice, no substance is entirely pure, and chemical purity is specified according to the intended use of the chemical. Chemical substances exist as solids, liquids, ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Zwikker Reagent
The Zwikker reagent is used as a simple spot-test to presumptively identify barbiturates. It is composed of a mixture of two solutions. Part A is 0.5 g of copper (II) sulfate in 100 ml of distilled water. Part B consists of 5% pyridine (v/v) in chloroform. One drop of each is added to the substance to be tested and any change in colour is observed. The test turns phenobarbital, pentobarbital and secobarbital light purple. Tea and tobacco turn yellow-green. The test's lack of specificity and tendency to produce false positives means it is not widely used for presumptive drug testing, although it does still play a role as a thin layer chromatography stain. It is named after the Dutch scientist Cornelis Zwikker. See also * Drug checking * Marquis reagent Marquis reagent is used as a simple spot-test to presumptively identify alkaloids as well as other compounds. It is composed of a mixture of formaldehyde and concentrated sulfuric acid, which is dripped onto the substance being te ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Simon's Reagent
Simon's reagent is used as a simple spot-test to presumptively identify alkaloids as well as other compounds. It reacts with secondary amines like MDMA and methamphetamine to give a blue solution. Uses The primary use of this reagent is for detecting secondary amines, such as MDMA and methamphetamine, and is typically used after the mecke or marquis reagents to differentiate between the two mentioned and amphetamine or MDA. Chemistry The reagent is typically provided in two parts: * A mixture of 2% sodium nitroprusside and 2% acetaldehyde in water (solution A) * A solution of 2% sodium carbonate in water (solution B) Separate storage of the aldehyde and base are necessary to prevent aldol polymerisation of the aldehyde. When exposed to an amine, reaction with acetaldehyde produces the enamine, which subsequently reacts with sodium nitroprusside to the imine. Finally, the iminium salt is hydrolysed to the bright blue Simon-Awe complex. Acetaldehyde can be replaced with ac ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Marquis Reagent
Marquis reagent is used as a simple spot-test to presumptively identify alkaloids as well as other compounds. It is composed of a mixture of formaldehyde and concentrated sulfuric acid, which is dripped onto the substance being tested. The United States Department of Justice method for producing the reagent is the addition of 100 mL of concentrated (95–98%) sulfuric acid to 5 mL of 40% formaldehyde. Different compounds produce different color reactions. Methanol may be added to slow down the reaction process to allow better observation of the colour change. It was first discovered in 1896Toxicology. Volume 2 : mechanisms and analytical methods — New York, New York ; San Francisco, California : Academic Press, 1961 — p. 247. and described by the Russian (Estonian) pharmacologist, [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Mandelin Reagent
The Mandelin reagent is used as a simple spot-test to presumptively identify alkaloids as well as other compounds. It is composed of a mixture of ammonium metavanadate and concentrated sulfuric acid. Its primary use is for the detection of ketamine and PMA Unlike the most common reagent test chemicals, it has a deep red colour that changes to yellow if there is no alkaloid, which occurs within about 48 hours of mixing. The United States Department of Justice method for producing the reagent is the addition of 100 mL of concentrated (95–98%) sulfuric acid to 0.5-1 g of Ammonium metavanadate. This reagent was invented by the German pharmacologist, Karl Friedrich Mandelin (1854–1906) at the University of Dorpat. See also *Drug checking * Dille–Koppanyi reagent * Folin's reagent * Froehde reagent *Liebermann reagent *Marquis reagent Marquis reagent is used as a simple spot-test to presumptively identify alkaloids as well as other compounds. It is composed of a mixture of f ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Liebermann Reagent
The Liebermann reagent named after Hungarian chemist Leo Liebermann (1852-1926) is used as a simple spot-test to presumptively identify alkaloids as well as other compounds. It is composed of a mixture of potassium nitrite and concentrated sulfuric acid. 1 g of potassium nitrite is used for every 10 mL of sulfuric acid. Potassium nitrite may also be substituted by sodium nitrite. It is used to test for cocaine, morphine, PMA and PMMA. The test is performed by scraping off a small amount of the substance and adding a drop of the reagent (which is initially clear and colorless). The results are analyzed by viewing the color of the resulting mixture, and by the time taken for the change in color to become apparent. See also *Drug checking Drug checking or pill testing is a way to reduce the harm from drug consumption by allowing users to find out the content and purity of substances that they intend to consume. This empowers users to make safer choices: to avoid more dangerous ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Froehde Reagent
The Froehde reagent is used as a simple spot-test to presumptively identify alkaloids, especially opioids, as well as other compounds. It is composed of a mixture of molybdic acid or a molybdate salt dissolved in hot, concentrated sulfuric acid, which is then dripped onto the substance being tested. The United States Department of Justice method for producing the reagent is the addition of 100 ml of hot, concentrated (95–98%) sulfuric acid to 0.5 g of sodium molybdate or molybdic acid. The Virginia Department of Forensic Science method uses 0.5 g ammonium molybdate per 100 ml H2SO4 (conc.) Unheated sulfuric acid can be used to prepare the reagent in a less dangerous manner, but 2–4 hours must be allowed for the molybdate to dissolve. See also *Reagent testing *Drug checking * Dille–Koppanyi reagent * Folin's reagent *Liebermann reagent *Mandelin reagent *Marquis reagent Marquis reagent is used as a simple spot-test to presumptively identify alkaloids as well as other c ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Folin's Reagent
Folin's reagent or sodium 1,2-naphthoquinone-4-sulfonate is a chemical reagent used as a derivatizing agent to measure levels of amines and amino acids. The reagent reacts with them in alkaline solution to produce a fluorescent material that can be easily detected. This should not be confused with Folin-Ciocalteu reagent, that is used to detect phenolic compounds. The Folin reagent can be used with an acidic secondary reagent to distinguish MDMA and related compounds from PMMA and related compounds. See also * Pill testing * Sullivan reaction * Dille–Koppanyi reagent *Froehde reagent *Liebermann reagent *Mandelin reagent *Marquis reagent *Mecke reagent The Mecke reagent is used as a simple spot-test to presumptively identify alkaloids as well as other compounds. It is composed of a mixture of selenous acid and concentrated sulfuric acid, which is dripped onto the substance being tested. The Unit ... * Simon's reagent * Zwikker reagent References {{reflist Biochemis ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Dille–Koppanyi Reagent
The Dille–Koppanyi reagent is used as a simple spot-test to presumptively identify barbiturates. It is composed of a mixture of two solutions. Part A is 0.1 g of cobalt(II) acetate dihydrate dissolved in 100 ml of methanol mixed with 0.2 ml of glacial acetic acid. Part B made up of is 5% isopropylamine (v/v) in methanol. Two drops of A are dropped onto the substance followed by one drop of B and any change in colour is observed. The test turns phenobarbital, pentobarbital, amobarbital and secobarbital light purple by complexation of cobalt with the barbiturate nitrogens. The test, in a slightly different formulation, was developed in the 1930s by the Hungarian-American pharmacologist Theodore Koppanyi (1901–1985) and the American Biochemist, James Madison Dille (1928–1986). See also * Drug checking * Marquis reagent * Froehde's reagent * Zwikker reagent The Zwikker reagent is used as a simple spot-test to presumptively identify barbiturates. It is composed of a mix ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Drug Checking
Drug checking or pill testing is a way to reduce the harm from drug consumption by allowing users to find out the content and purity of substances that they intend to consume. This empowers users to make safer choices: to avoid more dangerous substances, to use smaller quantities, and to avoid dangerous combinations. Drug checking services have developed over the last twenty-five years in twenty countries and are being considered in more countries, although attempts to implement them in some countries have been hindered by local laws. Drug checking initially focused on MDMA users in electronic dance music events but the services have broadened as drug use has become more complex. These developments have been strongly affected by local laws and culture, resulting in a diverse range of services, both for mobile services that attend events and festivals and fixed sites in town centres and entertainment districts. For instance, staff may or may not be able to handle illegal substances, ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |