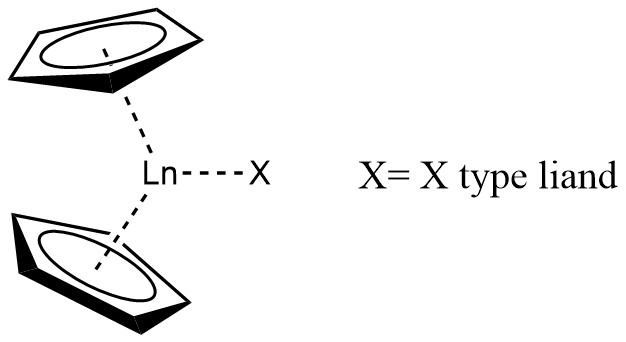|
Lanthanocene
A lanthanocene is a type of metallocene compound that contains an Chemical element, element from the lanthanide series. The most common lanthanocene complexes contain two Sodium cyclopentadienide, cyclopentadienyl anions and an Covalent bond classification method, X type ligand, usually hydride or Allyl ligand, alkyl ligand. History In 1954, Geoffrey Wilkinson, Wilkinson and Birmingham described the tris(cyclopentadienyl)lanthanide complex, Ln(C5H5)3 (Ln = Lanthanum, La, Cerium, Ce, Praseodymium, Pr, Neodymium, Nd, Samarium, Sm). However, due to the highly moisture and oxygen sensitive character of organolanthanide compounds, as well as the incapability of the separation of simple alkyl and aryl derivatives of the type LnR3, this area of organomemtallic chemistry experienced a period of relative stagnation for two decades before lanthanocene complexes were prepared for some of the later lanthanides (Ln = Gadolinium, Gd, Erbium, Er, Ytterbium, Yb, Lutetium, Lu). Synthesis The sy ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Lanthanocene Structure
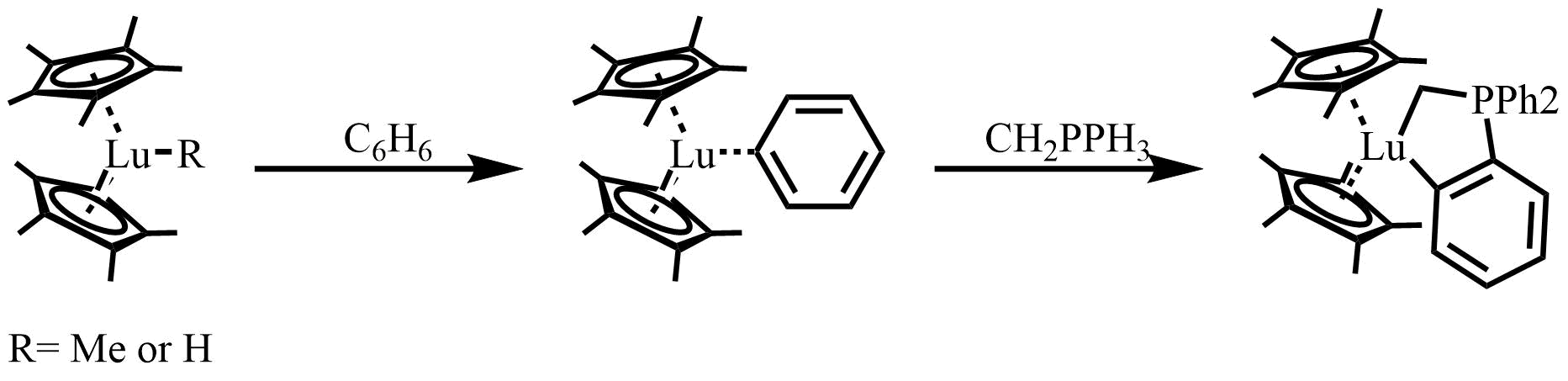
A lanthanocene is a type of metallocene compound that contains an element from the lanthanide series. The most common lanthanocene complexes contain two cyclopentadienyl anions and an X type ligand, usually hydride or alkyl ligand. History In 1954, Wilkinson and Birmingham described the tris(cyclopentadienyl)lanthanide complex, Ln(C5H5)3 (Ln = La, Ce, Pr, Nd, Sm). However, due to the highly moisture and oxygen sensitive character of organolanthanide compounds, as well as the incapability of the separation of simple alkyl and aryl derivatives of the type LnR3, this area of organomemtallic chemistry experienced a period of relative stagnation for two decades before lanthanocene complexes were prepared for some of the later lanthanides (Ln = Gd, Er, Yb, Lu). Synthesis The synthesis part will focus on lanthanide(III) metallocene complexes that contain Ln-C bonds, which are widely prepared from the corresponding Ln-Cl precursors as shown. : + LiR → + LiCl ::Ln = Y, ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Lanthanocene CH Activation 2
A lanthanocene is a type of metallocene compound that contains an element from the lanthanide series. The most common lanthanocene complexes contain two cyclopentadienyl anions and an X type ligand, usually hydride or alkyl ligand. History In 1954, Wilkinson Wilkinson may refer to: People * Wilkinson (surname), a surname (including a list of people with the name) Places in the United States * Wilkinson, Illinois * Wilkinson, Indiana, a town in Hancock County * Wilkinson, Minnesota * Wilkinson, Mis ... and Birmingham described the tris(cyclopentadienyl)lanthanide complex, Ln(C5H5)3 (Ln = La, Ce, Pr, Nd, Sm). However, due to the highly moisture and oxygen sensitive character of organolanthanide compounds, as well as the incapability of the separation of simple alkyl and aryl derivatives of the type LnR3, this area of organomemtallic chemistry experienced a period of relative stagnation for two decades before lanthanocene complexes were prepared for some of the later ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Lutetium
Lutetium is a chemical element with the symbol Lu and atomic number 71. It is a silvery white metal, which resists corrosion in dry air, but not in moist air. Lutetium is the last element in the lanthanide series, and it is traditionally counted among the rare earth elements. Lutetium is generally considered the first element of the 6th-period transition metals by those who study the matter, although there has been some dispute on this point. Lutetium was independently discovered in 1907 by French scientist Georges Urbain, Austrian mineralogist Baron Carl Auer von Welsbach, and American chemist Charles James. All of these researchers found lutetium as an impurity in the mineral ytterbia, which was previously thought to consist entirely of ytterbium. The dispute on the priority of the discovery occurred shortly after, with Urbain and Welsbach accusing each other of publishing results influenced by the published research of the other; the naming honor went to Urbain, as he had publis ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Hydrogenation Mechanism
Hydrogenation is a chemical reaction between molecular hydrogen (H2) and another compound or element, usually in the presence of a catalyst such as nickel, palladium or platinum. The process is commonly employed to reduce or saturate organic compounds. Hydrogenation typically constitutes the addition of pairs of hydrogen atoms to a molecule, often an alkene. Catalysts are required for the reaction to be usable; non-catalytic hydrogenation takes place only at very high temperatures. Hydrogenation reduces double and triple bonds in hydrocarbons. Process Hydrogenation has three components, the unsaturated substrate, the hydrogen (or hydrogen source) and, invariably, a catalyst. The reduction reaction is carried out at different temperatures and pressures depending upon the substrate and the activity of the catalyst. Related or competing reactions The same catalysts and conditions that are used for hydrogenation reactions can also lead to isomerization of the alkenes from cis to ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Sigma-bond Metathesis
In organometallic chemistry, sigma-bond metathesis is a chemical reaction wherein a metal-ligand sigma bond undergoes metathesis (exchange of parts) with the sigma bond in some reagent. The reaction is illustrated by the exchange of lutetium(III) methyl complex with a hydrocarbon (R-H): :(C5Me5)2Lu-CH3 + R-H → (C5Me5)2Lu-R + CH4 This reactivity was first observed by Patricia Watson, a researcher at duPont.{{cite journal, author=Watson, Patricia, title=Methane exchange reactions of lanthanide and early-transition-metal methyl complexes, journal=Journal of the American Chemical Society, year=1983, volume=32, pages=6491–6493, doi=10.1021/ja00359a023 The reaction is mainly observed for complexes of metals with d0 configuration, e.g. complexes of Sc(III), Zr(IV), Nb(IV), Ta(V), etc. f-Element complexes also participate, regardless of the number of f-electrons. The reaction is thought to proceed via cycloaddition. Indeed, the rate of the reaction is characterized by a highly ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Lan Hydrofunctionalization Png
Lan or LAN may also refer to: Science and technology * Local asymptotic normality, a fundamental property of regular models in statistics * Longitude of the ascending node, one of the orbital elements used to specify the orbit of an object in space * Łan, unit of measurement in Poland * Local area network, a computer network that interconnects within a limited area such as one or more buildings * Lan blood group system, a human blood group Places * Lancashire (Chapman code), England * Lancaster railway station (National Rail station code), England * Capital Region International Airport (IATA airport code), Lansing, Michigan, US * Lan County, Shanxi, China * Łan, Lublin Voivodeship, Poland * Lan (river), Belarus * Llan (placename), a placename element known in Breton as ''lan'' Airlines * LAN Airlines, former name of LATAM Chile, an airline in Chile, with a stake in other airlines: ** LAN Peru, an airline based in Peru ** LAN Ecuador, an airline based in Quito, Ecuador ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Hydroamination
In organic chemistry, hydroamination is the addition of an bond of an amine across a carbon-carbon multiple bond of an alkene, alkyne, diene, or allene. In the ideal case, hydroamination is atom economical and green. Amines are common in fine-chemical, pharmaceutical, and agricultural industries. Hydroamination can be used intramolecularly to create heterocycles or intermolecularly with a separate amine and unsaturated compound. The development of catalysts for hydroamination remains an active area, especially for alkenes. Although practical hydroamination reactions can be effected for dienes and electrophilic alkenes, the term hydroamination often implies reactions metal-catalyzed processes. History Hydroamination is well-established technology for generating fragrances from myrcene. In this conversion, diethylamine adds across the diene substituent, the reaction being catalyzed by lithium diethylamide. Intramolecular hydroaminations were reported by Tobin J. Marks in 1989 ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Hydroboration
In organic chemistry, hydroboration refers to the addition of a hydrogen-boron bond to certain double and triple bonds involving carbon (, , , and ). This chemical reaction is useful in the organic synthesis of organic compounds. Hydroboration produces organoborane compounds that react with a variety of reagents to produce useful compounds, such as alcohols, amines, or alkyl halides. The most widely known reaction of the organoboranes is oxidation to produce alcohols typically by hydrogen peroxide. This type of reaction has promoted research on hydroboration because of its mild condition and a wide scope of tolerated alkenes. Another research subtheme is metal-catalysed hydroboration. The development of this technology and the underlying concepts were recognized by the Nobel Prize in Chemistry to Herbert C. Brown. He shared the prize with Georg Wittig in 1979 for his pioneering research on organoboranes as important synthetic intermediates. Addition of a H-B bond to C-C doubl ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Hydrosilylation
Hydrosilylation, also called catalytic hydrosilation, describes the addition of Si-H bonds across unsaturated bonds."Hydrosilylation A Comprehensive Review on Recent Advances" B. Marciniec (ed.), Advances in Silicon Science, Springer Science, 2009. Ordinarily the reaction is conducted catalytically and usually the substrates are unsaturated organic compounds. Alkenes and alkynes give alkyl and vinyl silanes; aldehydes and ketones give silyl ethers. Hydrosilylation has been called the "most important application of platinum in homogeneous catalysis." Scope and mechanism Hydrosilylation of alkenes represents a commercially important method for preparing organosilicon compounds. The process is mechanistically similar to the hydrogenation of alkenes. In fact, similar catalysts are sometimes employed for the two catalytic processes. The prevalent mechanism, called the Chalk-Harrod mechanism, assumes an intermediate metal complex that contains a hydride, a silyl ligand (R3Si), and ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Hydrogenation
Hydrogenation is a chemical reaction between molecular hydrogen (H2) and another compound or element, usually in the presence of a Catalysis, catalyst such as nickel, palladium or platinum. The process is commonly employed to redox, reduce or Saturated and unsaturated compounds, saturate organic compounds. Hydrogenation typically constitutes the addition of pairs of hydrogen atoms to a molecule, often an alkene. Catalysts are required for the reaction to be usable; non-catalytic hydrogenation takes place only at very high temperatures. Hydrogenation reduces Double bond, double and Triple bond, triple bonds in hydrocarbons. Process Hydrogenation has three components, the Saturated and unsaturated compounds, unsaturated substrate, the hydrogen (or hydrogen source) and, invariably, a catalyst. The redox, reduction reaction is carried out at different temperatures and pressures depending upon the substrate and the activity of the catalyst. Related or competing reactions The same ca ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Hydrofunctionalization
A hydrofunctionalization reaction is the addition of hydrogen and another univalent fragment (X) across a carbon-carbon or carbon-heteroatom multiple bond. Often, the term ''hydrofunctionalization'' without modifier refers specifically to the use of the covalent hydride (H-X) as the source of hydrogen and X for this transformation. If other reagents are used to achieve the net addition of hydrogen and X across a multiple bond, the process may be referred to as a ''formal hydrofunctionalization''. For terminal olefins (or acetylenes), the regioselectivity of the process can be described as Markovnikov (addition of X at the substituted end) or anti-Markovnikov (addition of X at the unsubstituted end). Catalysts are frequently employed to control the chemo-, regio-, and stereoselectivity of hydrofunctionalization reactions. Examples Some of the better known classes of hydrofunctionalization reactions include the following: *Hydroboration *Hydrosilylation *Hydrometalation (incl ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Ylide
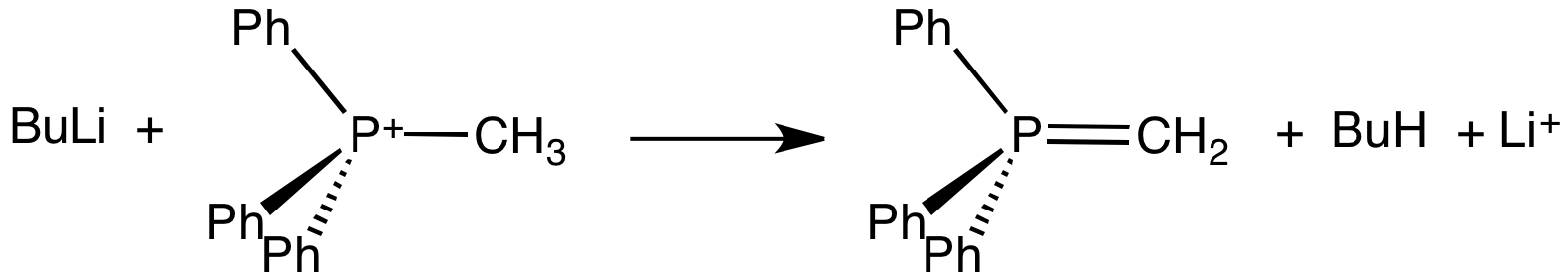
An ylide or ylid () is a neutral dipolar molecule containing a formally negatively charged atom (usually a carbanion) directly attached to a heteroatom with a formal positive charge (usually nitrogen, phosphorus or sulfur), and in which both atoms have full octets of electrons. The result can be viewed as a structure in which two adjacent atoms are connected by both a covalent and an ionic bond; normally written X+–Y−. Ylides are thus 1,2-dipolar compounds, and a subclass of zwitterions. They appear in organic chemistry as reagents or reactive intermediates. The class name "ylide" for the compound should not be confused with the suffix "-ylide". Resonance structures Many ylides may be depicted by a multiple bond form in a resonance structure, known as the ylene form, while the actual structure lies in between both forms: : The actual bonding picture of these types of ylides is strictly zwitterionic (the structure on the right) with the strong Coulombic attraction between the " ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |