|
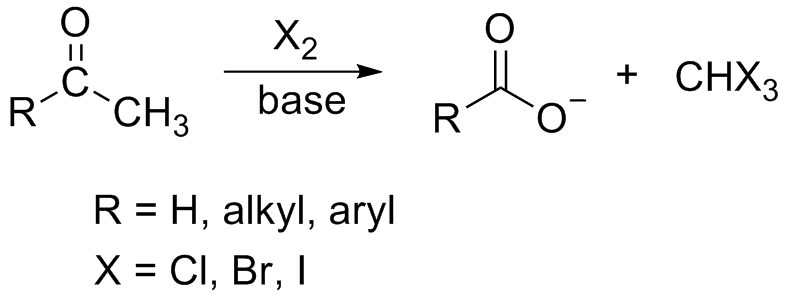
Iodoform Test
In chemistry, the haloform reaction is a chemical reaction in which a haloform (, where X is a halogen) is produced by the exhaustive halogenation of an acetyl group (, where R can be either a hydrogen atom, an alkyl or an aryl group), in the presence of a base. The reaction can be used to transform acetyl groups into carboxyl groups () or to produce chloroform (), bromoform (), or iodoform (). Note that fluoroform () can't be prepared in this way. Mechanism In the first step, the halogen dis-proportionates in the presence of hydroxide to give the halide and hypohalite. :Br2 + 2 OH- -> Br- + BrO- + H2O If a secondary alcohol is present, it is oxidized to a ketone by the hypohalite: If a methyl ketone is present, it reacts with the hypohalite in a three-step process: 1. Under basic conditions, the ketone undergoes keto-enol tautomerisation. The enolate undergoes electrophilic attack by the hypohalite (containing a halogen with a formal +1 charge). : 2. When the α( ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Adolf Lieben
Adolf Lieben (3 December 1836 – 6 June 1914) was an Austrian Jewish chemist. He was born in Vienna the son of Ignatz Lieben. He studied at the University of Vienna, University of Heidelberg (Ph.D. 1856 with Robert Wilhelm Bunsen), and Paris, and subsequently held the positions of privat-docent at the University of Vienna (1861), and professor in the universities of Palermo (1863), Turin (1867), and Prague (1871). From 1875 until his death he held the chair of general and pharmacological chemistry at the University of Vienna, and is a member of the Vienna Academy of Sciences. Publications Lieben has published many essays in '' Liebig's Annalen der Chemie'': *"Ueber die Einwirkung schwacher Affinitäten auf Aldehyde," 1861; *"Ueber das Iodbenzol," 1869; *"Ueber festes Benzoylchlorid," 1875; etc., *"Sitzungsberichte den Kaiserlichen Akademie der Wissenschaften in Wien" ("Untersuchungen über Milchzucker," "Einwirkung von Cyangas auf Aldehyde," "Ueber den Formaldehyd und desse ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Bromoform 1
Bromoform (CHBr3) is a brominated organic solvent, colorless liquid at room temperature, with a high refractive index, very high density, and sweet odor is similar to that of chloroform. It is one of the four haloforms, the others being fluoroform, chloroform, and iodoform. Bromoform can be prepared by the haloform reaction using acetone and sodium hypobromite, by the electrolysis of potassium bromide in ethanol, or by treating chloroform with aluminium bromide. Currently its main use is as a laboratory reagent. Structure The molecule adopts tetrahedral molecular geometry with C3v symmetry. Uses Only small quantities of bromoform are currently produced industrially in the United States. In the past, it was used as a solvent, sedative and flame retardant, but now it is mainly used as a laboratory reagent, for example as an extraction solvent. Bromoform also has medical uses; injections of bromoform are sometimes used instead of epinephrine to treat severe asthma cases. Bromoform' ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Acetoacetic Acid
Acetoacetic acid (also acetoacetate and diacetic acid) is the organic compound with the formula CHCOCHCOOH. It is the simplest beta-keto acid, and like other members of this class, it is unstable. The methyl and ethyl esters, which are quite stable, are produced on a large scale industrially as precursors to dyes. Acetoacetic acid is a weak acid. Biochemistry Under typical physiological conditions, acetoacetic acid exists as its conjugate base, acetoacetate. Acetoacetate is produced in the mitochondria of the liver from acetoacetyl coenzyme A (CoA). First, another acetyl group is added from acetyl CoA to form 3-hydroxy-3-methylglutaryl CoA, then an acetyl CoA is lost from this, yielding acetoacetate. The initial acetoacetate can come from the last cycle in the beta oxidation of a fatty acid, or it can be synthesized from two acetyl CoA molecules, catalyzed by thiolase. In mammals, acetoacetate produced in the liver (along with the other two " ketone bodies") is released into ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Acetylacetone
Acetylacetone is an organic compound with the chemical formula . It is a colorless liquid, classified as a 1,3-diketone. It exists in equilibrium with a tautomer . These tautomers interconvert so rapidly under most conditions that they are treated as a single compound in most applications. It is a colorless liquid that is a precursor to acetylacetonate anion (commonly abbreviated acac−), a bidentate ligand. It is also a building block for the synthesis of heterocyclic compounds. Properties Tautomerism The keto and enol tautomers of acetylacetone coexist in solution. The enol form has C2v symmetry, meaning the hydrogen atom is shared equally between the two oxygen atoms. In the gas phase, the equilibrium constant, ''K''keto→enol, is 11.7, favoring the enol form. The two tautomeric forms can be distinguished by NMR spectroscopy, IR spectroscopy and other methods. The equilibrium constant tends to be high in nonpolar solvents; when k = >1, the enol form is favour ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Acetaldehyde
Acetaldehyde (IUPAC systematic name ethanal) is an organic chemical compound with the formula CH3 CHO, sometimes abbreviated by chemists as MeCHO (Me = methyl). It is a colorless liquid or gas, boiling near room temperature. It is one of the most important aldehydes, occurring widely in nature and being produced on a large scale in industry. Acetaldehyde occurs naturally in coffee, bread, and ripe fruit, and is produced by plants. It is also produced by the partial oxidation of ethanol by the liver enzyme alcohol dehydrogenase and is a contributing cause of hangover after alcohol consumption. Pathways of exposure include air, water, land, or groundwater, as well as drink and smoke. Consumption of disulfiram inhibits acetaldehyde dehydrogenase, the enzyme responsible for the metabolism of acetaldehyde, thereby causing it to build up in the body. The International Agency for Research on Cancer (IARC) has listed acetaldehyde as a Group 1 carcinogen. Acetaldehyde is "one of the mos ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Ethanol
Ethanol (abbr. EtOH; also called ethyl alcohol, grain alcohol, drinking alcohol, or simply alcohol) is an organic compound. It is an Alcohol (chemistry), alcohol with the chemical formula . Its formula can be also written as or (an ethyl group linked to a hydroxyl group). Ethanol is a Volatility (chemistry), volatile, Combustibility and flammability, flammable, colorless liquid with a characteristic wine-like odor and pungent taste. It is a psychoactive recreational drug, the active ingredient in alcoholic drinks. Ethanol is naturally produced by the fermentation process of Carbohydrate, sugars by yeasts or via Petrochemistry, petrochemical processes such as ethylene hydration. It has medical applications as an antiseptic and disinfectant. It is used as a chemical solvent and in the Chemical synthesis, synthesis of organic compounds, and as a Alcohol fuel, fuel source. Ethanol also can be dehydrated to make ethylene, an important chemical feedstock. As of 2006, world produ ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Aldehyde
In organic chemistry, an aldehyde () is an organic compound containing a functional group with the structure . The functional group itself (without the "R" side chain) can be referred to as an aldehyde but can also be classified as a formyl group. Aldehydes are common and play important roles in the technology and biological spheres. Structure and bonding Aldehydes feature a carbon center that is connected by a double bond to oxygen and a single bond to hydrogen and single bond to a third substituent, which is carbon or, in the case of formaldehyde, hydrogen. The central carbon is often described as being sp2- hybridized. The aldehyde group is somewhat polar. The C=O bond length is about 120-122 picometers. Physical properties and characterization Aldehydes have properties that are diverse and that depend on the remainder of the molecule. Smaller aldehydes are more soluble in water, formaldehyde and acetaldehyde completely so. The volatile aldehydes have pungent odors. Al ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Isopropanol
Isopropyl alcohol (IUPAC name propan-2-ol and also called isopropanol or 2-propanol) is a colorless, flammable organic compound with a pungent alcoholic odor. As an isopropyl group linked to a hydroxyl group (chemical formula ) it is the simplest example of a secondary alcohol, where the alcohol carbon atom is attached to two other carbon atoms. It is a structural isomer of propan-1-ol and ethyl methyl ether. It is used in the manufacture of a wide variety of industrial and household chemicals and is a common ingredient in products such as antiseptics, disinfectants, hand sanitizer and detergents. Well over one million tonnes is produced worldwide annually. Properties Isopropyl alcohol is miscible in water, ethanol, and chloroform as, like these compounds, isopropyl is a polar molecule. It dissolves ethyl cellulose, polyvinyl butyral, many oils, alkaloids, and natural resins. Unlike ethanol or methanol, isopropyl alcohol is not miscible with salt solutions and can be se ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Alcohol (chemistry)
In chemistry, an alcohol is a type of organic compound that carries at least one hydroxyl () functional group bound to a saturated carbon atom. The term ''alcohol'' originally referred to the primary alcohol ethanol (ethyl alcohol), which is used as a drug and is the main alcohol present in alcoholic drinks. An important class of alcohols, of which methanol and ethanol are the simplest examples, includes all compounds which conform to the general formula . Simple monoalcohols that are the subject of this article include primary (), secondary () and tertiary () alcohols. The suffix ''-ol'' appears in the IUPAC chemical name of all substances where the hydroxyl group is the functional group with the highest priority. When a higher priority group is present in the compound, the prefix ''hydroxy-'' is used in its IUPAC name. The suffix ''-ol'' in non-IUPAC names (such as paracetamol or cholesterol) also typically indicates that the substance is an alcohol. However, some compou ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Haloform Schritt 2
In chemistry, trihalomethanes (THMs) are chemical compounds in which three of the four hydrogen atoms of methane () are replaced by halogen atoms. Many trihalomethanes find uses in industry as solvents or refrigerants. THMs are also environmental pollutants, and many are considered carcinogenic. Trihalomethanes with all the same halogen atoms are called haloforms. Table of common trihalomethanes Industrial uses Only chloroform has significant applications of the haloforms. In the predominant application, chloroform is required for the production of tetrafluoroethylene, precursor to teflon. Chloroform is fluorinated by reaction with hydrogen fluoride to produce chlorodifluoromethane (R-22). Pyrolysis of chlorodifluoromethane (at 550-750 °C) yields TFE, with difluorocarbene as an intermediate. :CHCl3 + 2 HF -> CHClF2 + 2 HCl :2 CHClF2 -> C2F4 + 2 HCl Refrigerants and solvents Trifluoromethane and chlorodifluoromethane are both used as refrigerants. Trihalomethanes ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Chloral Hydrate
Chloral hydrate is a geminal diol with the formula . It is a colorless solid. It has limited use as a sedative and hypnotic pharmaceutical drug. It is also a useful laboratory chemical reagent and precursor. It is derived from chloral (trichloroacetaldehyde) by the addition of one equivalent of water. History Chloral hydrate was discovered in 1832 by Justus von Liebig in Gießen when a chlorination ( halogenation) reaction was performed on ethanol. Its sedative properties were observed by Rudolf Buchheim in 1861, but described in detail and published only in 1869 by Oscar Liebreich; subsequently, because of its easy synthesis, its use became widespread. It was widely used for sedation in asylums and in general medical practice, and also became a popular drug of abuse in the late 19th century. One recreational user was the poet and illustrator Dante Gabriel Rossetti. Chloral hydrate is soluble in both water and ethanol, readily forming concentrated solutions. A solution of c ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Electron-withdrawing Group
In chemistry, an electron-withdrawing group (EWG) is a substituent that has some of the following kinetic and thermodynamic implications: *with regards to electron transfer, electron-withdrawing groups enhance the oxidizing power tendency of the appended species. Tetracyanoethylene is an oxidant because the alkene is appended to four cyano substituents, which are electron-withdrawing. *with regards to acid-base reactions, acids with electron-withdrawing groups species have low acid dissociation constants. For EWG's attached to benzene, this effect is described by the Hammett equation, which allows EWGs to be discussed quantitatively. *with regards to nucleophilic substitution reactions, electron-withdrawing groups are susceptible to attack by weak nucleophiles. For example, compared to chlorobenzene, chlorodinitrobenzene is susceptible to reactions that displace chloride.{{cite journal , author=J. F. Bunnett, R. M. Conner, doi=10.15227/orgsyn.040.0034, title=2,4-Dinitroiodobe ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |