|
Indium(III) Oxide
Indium(III) oxide ( In2 O3) is a chemical compound, an amphoteric oxide of indium. Physical properties Crystal structure Amorphous indium oxide is insoluble in water but soluble in acids, whereas crystalline indium oxide is insoluble in both water and acids. The crystalline form exists in two phases, the cubic ( bixbyite type) and rhombohedral ( corundum type). Both phases have a band gap of about 3 eV. The parameters of the cubic phase are listed in the infobox. The rhombohedral phase is produced at high temperatures and pressures or when using non-equilibrium growth methods. It has a space group Rc No. 167, Pearson symbol hR30, a = 0.5487 nm, b = 0.5487 nm, c = 1.4510 nm, Z = 6 and calculated density 7.31 g/cm3. Conductivity and magnetism Thin films of chromium- doped indium oxide (In2−xCrxO3) are a magnetic semiconductor displaying high-temperature ferromagnetism, single- phase crystal structure, and semiconductor behavior with high concentration of charge ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Bixbyite
Bixbyite is a manganese iron oxide mineral with chemical formula: (Mn,Fe)2O3. The iron/manganese ratio is quite variable and many specimens have almost no iron. It is a metallic dark black with a Mohs hardness of 6.0 - 6.5. It is a somewhat rare mineral sought after by collectors as it typically forms euhedral isometric crystals exhibiting various cubes, octahedra, and dodecahedra. It is commonly associated with beryl, quartz, spessartine, hematite, pseudobrookite, hausmannite, braunite and topaz in pneumatolytic or hydrothermal veins and cavities and in metamorphic rocks. It can also be found in lithophysal cavities in rhyolite. Typical localities are Jhabua and Chhindwara districts, India and the Thomas Range in Juab County, Utah. It is also reported from San Luis Potosi, Mexico; northern Patagonia, Argentina; Girona, Catalonia, Spain; Sweden, Germany, Namibia, Zimbabwe, and South Africa. Bixbyite was named for the American mineralogist Maynard Bixby (1853–1935), respons ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Semiconductor
A semiconductor is a material which has an electrical conductivity value falling between that of a conductor, such as copper, and an insulator, such as glass. Its resistivity falls as its temperature rises; metals behave in the opposite way. Its conducting properties may be altered in useful ways by introducing impurities ("doping") into the crystal structure. When two differently doped regions exist in the same crystal, a semiconductor junction is created. The behavior of charge carriers, which include electrons, ions, and electron holes, at these junctions is the basis of diodes, transistors, and most modern electronics. Some examples of semiconductors are silicon, germanium, gallium arsenide, and elements near the so-called "metalloid staircase" on the periodic table. After silicon, gallium arsenide is the second-most common semiconductor and is used in laser diodes, solar cells, microwave-frequency integrated circuits, and others. Silicon is a critical element for ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Aluminium
Aluminium (aluminum in AmE, American and CanE, Canadian English) is a chemical element with the Symbol (chemistry), symbol Al and atomic number 13. Aluminium has a density lower than those of other common metals, at approximately one third that of steel. It has a great affinity towards oxygen, and Passivation (chemistry), forms a protective layer of Aluminium oxide, oxide on the surface when exposed to air. Aluminium visually resembles silver, both in its color and in its great ability to reflect light. It is soft, Magnetism, non-magnetic and ductility, ductile. It has one stable isotope, 27Al; this isotope is very common, making aluminium the twelfth most common element in the Universe. The radioactivity of Aluminum-26, 26Al is used in Radiometric dating, radiodating. Chemically, aluminium is a post-transition metal in the boron group; as is common for the group, aluminium forms compounds primarily in the +3 oxidation state. The aluminium cation Al3+ is small and h ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Diffusion
Diffusion is the net movement of anything (for example, atoms, ions, molecules, energy) generally from a region of higher concentration to a region of lower concentration. Diffusion is driven by a gradient in Gibbs free energy or chemical potential. It is possible to diffuse "uphill" from a region of lower concentration to a region of higher concentration, like in spinodal decomposition. The concept of diffusion is widely used in many fields, including physics (particle diffusion), chemistry, biology, sociology, economics, and finance (diffusion of people, ideas, and price values). The central idea of diffusion, however, is common to all of these: a substance or collection undergoing diffusion spreads out from a point or location at which there is a higher concentration of that substance or collection. A gradient is the change in the value of a quantity, for example, concentration, pressure, or temperature with the change in another variable, usually distance. A change in c ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Barrier Metal
A diffusion barrier is a thin layer (usually micrometres thick) of metal usually placed between two other metals. It is done to act as a barrier to protect either one of the metals from corrupting the other.. Adhesion of a plated metal layer to its substrate requires a physical interlocking, inter-diffusion of the deposit or a chemical bonding between plate and substrate in order to work. The role of a diffusion barrier is to prevent or to retard the inter-diffusion of the two superposed metals. Therefore, to be effective, a good diffusion barrier requires inertness with respect to adjacent materials. To obtain good adhesion and a diffusion barrier simultaneously, the bonding between layers needs to come from a chemical reaction of limited range at both boundaries. Materials providing good adhesion are not necessarily good diffusion barriers and vice versa. Consequently, there are cases where two or more separate layers must be used to provide a proper interface between substrates. ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Oxygen
Oxygen is the chemical element with the symbol O and atomic number 8. It is a member of the chalcogen group in the periodic table, a highly reactive nonmetal, and an oxidizing agent that readily forms oxides with most elements as well as with other compounds. Oxygen is Earth's most abundant element, and after hydrogen and helium, it is the third-most abundant element in the universe. At standard temperature and pressure, two atoms of the element bind to form dioxygen, a colorless and odorless diatomic gas with the formula . Diatomic oxygen gas currently constitutes 20.95% of the Earth's atmosphere, though this has changed considerably over long periods of time. Oxygen makes up almost half of the Earth's crust in the form of oxides.Atkins, P.; Jones, L.; Laverman, L. (2016).''Chemical Principles'', 7th edition. Freeman. Many major classes of organic molecules in living organisms contain oxygen atoms, such as proteins, nucleic acids, carbohydrates, and fats ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Argon
Argon is a chemical element with the symbol Ar and atomic number 18. It is in group 18 of the periodic table and is a noble gas. Argon is the third-most abundant gas in Earth's atmosphere, at 0.934% (9340 ppmv). It is more than twice as abundant as water vapor (which averages about 4000 ppmv, but varies greatly), 23 times as abundant as carbon dioxide (400 ppmv), and more than 500 times as abundant as neon (18 ppmv). Argon is the most abundant noble gas in Earth's crust, comprising 0.00015% of the crust. Nearly all of the argon in Earth's atmosphere is radiogenic argon-40, derived from the decay of potassium-40 in Earth's crust. In the universe, argon-36 is by far the most common argon isotope, as it is the most easily produced by stellar nucleosynthesis in supernovas. The name "argon" is derived from the Greek word , neuter singular form of meaning 'lazy' or 'inactive', as a reference to the fact that the element undergoes almost no chemical reactions. The comp ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
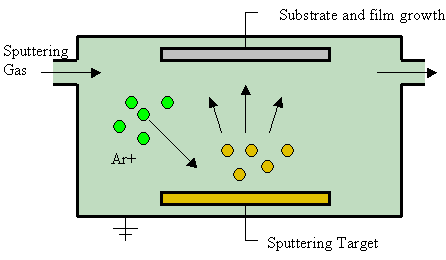
Sputter Deposition
Sputter deposition is a physical vapor deposition (PVD) method of thin film deposition by the phenomenon of sputtering. This involves ejecting material from a "target" that is a source onto a "substrate" such as a silicon wafer. Resputtering is re-emission of the deposited material during the deposition process by ion or atom bombardment. Sputtered atoms ejected from the target have a wide energy distribution, typically up to tens of eV (100,000 K). The sputtered ions (typically only a small fraction of the ejected particles are ionized — on the order of 1 percent) can ballistically fly from the target in straight lines and impact energetically on the substrates or vacuum chamber (causing resputtering). Alternatively, at higher gas pressures, the ions collide with the gas atoms that act as a moderator and move diffusively, reaching the substrates or vacuum chamber wall and condensing after undergoing a random walk. The entire range from high-energy ballistic impact to low-en ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Indium(III) Hydroxide
Indium(III) hydroxide is the chemical compound with the formula In(OH)3. Its prime use is as a precursor to indium(III) oxide, In2O3. It is sometimes found as the rare mineral dzhalindite. Structure Indium(III) hydroxide has a cubic structure, space group Im3, a distorted ReO3 structure. Preparation and reactions Neutralizing a solution containing an In3+ salt such as indium nitrate (In(NO3)3) or a solution of indium trichloride (InCl3) gives a white precipitate that on aging forms indium(III) hydroxide. A thermal decomposition of freshly prepared In(OH)3 shows the first step is the conversion of In(OH)3xH2O to cubic indium(III) hydroxide. The precipitation of indium hydroxide was a step in the separation of indium from zincblende ore by Reich and Richter, the discoverers of indium. Indium(III) hydroxide is amphoteric, like gallium(III) hydroxide (Ga(OH)3) and aluminium hydroxide (Al(OH)3), but is much less acidic than gallium hydroxide (Ga(OH)3), having a lower solu ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Transition Temperature
Transition temperature is the temperature at which a material changes from one crystal state ( allotrope) to another. More formally, it is the temperature at which two crystalline forms of a substance can co-exist in equilibrium. For example, when rhombic sulfur is heated above 95.6 °C, it changes form into monoclinic sulfur; when cooled below 95.6 °C, it reverts to rhombic sulfur. At 95.6 °C the two forms can co-exist. Another example is tin, which transitions from a cubic crystal below 13.2 °C to a tetragonal crystal above that temperature. In the case of ferroelectric or ferromagnetic crystals, a transition temperature may be known as the Curie temperature. See also * Crystal system In crystallography, a crystal system is a set of point groups (a group of geometric symmetries with at least one fixed point). A lattice system is a set of Bravais lattices. Space groups are classified into crystal systems according to their po ... Crystallogra ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Liquid Helium
Liquid helium is a physical state of helium at very low temperatures at standard atmospheric pressures. Liquid helium may show superfluidity. At standard pressure, the chemical element helium exists in a liquid form only at the extremely low temperature of . Its boiling point and critical point depend on which isotope of helium is present: the common isotope helium-4 or the rare isotope helium-3. These are the only two stable isotopes of helium. See the table below for the values of these physical quantities. The density of liquid helium-4 at its boiling point and a pressure of one atmosphere (101.3 kilopascals) is about , or about one-eighth the density of liquid water. Liquefaction Helium was first liquefied on July 10, 1908, by the Dutch physicist Heike Kamerlingh Onnes at the University of Leiden in the Netherlands. At that time, helium-3 was unknown because the mass spectrometer had not yet been invented. In more recent decades, liquid helium has been used as a ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |