|
Indene
Indene is a flammable polycyclic hydrocarbon with chemical formula . It is composed of a benzene ring fused with a cyclopentene ring. This aromatic liquid is colorless although samples often are pale yellow. The principal industrial use of indene is in the production of indene/coumarone thermoplastic resins. Substituted indenes and their closely related indane derivatives are important structural motifs found in many natural products and biologically active molecules, such as sulindac. Isolation Indene occurs naturally in coal-tar fractions boiling around 175–185 °C. It can be obtained by heating this fraction with sodium to precipitate solid "sodio-indene". This step exploits indene's weak acidity evidenced by its deprotonation by sodium to give the indenyl derivative. The sodio-indene is converted back to indene by steam distillation. Reactivity Indene readily polymerises. Oxidation of indene with acid dichromate yields homophthalic acid (''o''-carboxylphenylace ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Sulindac
Sulindac is a nonsteroidal anti-inflammatory drug (NSAID) of the arylalkanoic acid class that is marketed as Clinoril. Imbaral (not to be confused with mebaral) is another name for this drug. Its name is derived from sul(finyl)+ ind(ene)+ ac(etic acid) It was patented in 1969 and approved for medical use in 1976. Medical uses Like other NSAIDs, it is useful in the treatment of acute or chronic inflammatory conditions. Sulindac is a prodrug, derived from sulfinylindene, that is converted in the body to the active NSAID. More specifically, the agent is converted by liver enzymes to a sulfide that is excreted in the bile and then reabsorbed from the intestine. This is thought to help maintain constant blood levels with reduced gastrointestinal side effects. Some studies have shown sulindac to be relatively less irritating to the stomach than other NSAIDs except for drugs of the COX-2 inhibitor class . The exact mechanism of its NSAID properties is unknown, but it is thought to a ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
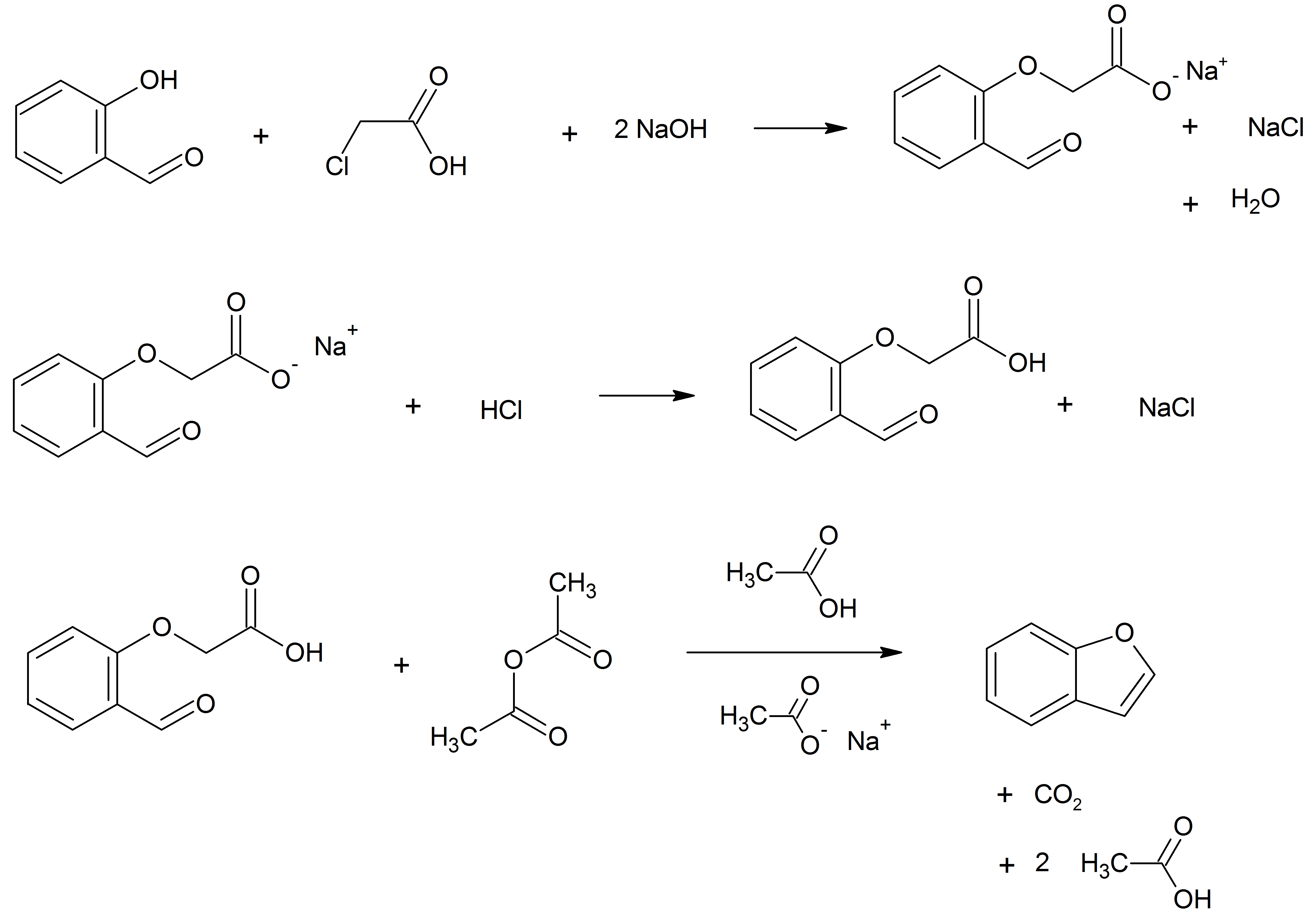
Benzofuran
Benzofuran is the heterocyclic compound consisting of fused benzene and furan rings. This colourless liquid is a component of coal tar. Benzofuran is the "parent" of many related compounds with more complex structures. For example, psoralen is a benzofuran derivative that occurs in several plants. Production Benzofuran is extracted from coal tar. It is also obtained by dehydrogenation of 2-ethylphenol. Laboratory methods Benzofurans can be prepared by various methods in the laboratory. Notable examples include: *''O''-alkylation of salicylaldehyde with chloroacetic acid followed by dehydration (cyclication) of the resulting ether and decarboxylation. *Perkin rearrangement, where a coumarin is reacted with a hydroxide: : * Diels–Alder reaction of nitro vinyl furans with various dienophiles: : Diels–Alder reaction yielding a substituted benzofuran, 450px * Cycloisomerization of alkyne ortho-substituted phenols: : Benzofurans via Cycloisomerization, 400px Related com ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Benzothiophene
Benzothiophene is an aromatic organic compound with a molecular formula C8H6S and an odor similar to naphthalene (mothballs). It occurs naturally as a constituent of petroleum-related deposits such as lignite tar. Benzothiophene has no household use. In addition to benzo hiophene, a second isomer is known: benzo hiophene. Benzothiophene finds use in research as a starting material for the synthesis of larger, usually bioactive structures. It is found within the chemical structures of pharmaceutical drugs such as raloxifene, zileuton, and sertaconazole, and also BTCP. It is also used in the manufacturing of dyes such as thioindigo. Synthesis Most syntheses of benzothiophene create substituted benzothiophenes as a precursor to further reactions. An example is the reaction of an alkyne-substituted 2-bromobenzene with either sodium sulphide or potassium sulphide to form benzothiophene with an alkyl substitution at position 2. Thiourea Thiourea () is an organosulfur co ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Indane
Indane or indan is an organic compound with the formula C6H4(CH2)3. It is a colorless liquid hydrocarbon. It is a petrochemical, a bicyclic compound. It occurs at the level of about 0.1% in coal tar. It is usually produced by hydrogenation of indene.Karl Griesbaum, Arno Behr, Dieter Biedenkapp, Heinz-Werner Voges, Dorothea Garbe, Christian Paetz, Gerd Collin, Dieter Mayer, Hartmut Höke "Hydrocarbons" in Ullmann's Encyclopedia of Industrial Chemistry 2002 Wiley-VCH, Weinheim. Reactions Indane can also be converted in a catalytic reactor to other aromatics such as xylene. Indane is used in the synthesis of sulofenur. Derivatives Derivatives include compounds such as 1-methylindane and 2-methylindane (where one methyl group is attached to the five carbon ring), 4-methylindane and 5-methylindane (where one methyl group is attached to the benzene ring), and various dimethylindanes. Other derivatives can be obtained indirectly, e.g. the reaction of diethyl phthalate with ethyl ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Coumarone
Benzofuran is the heterocyclic compound consisting of fused benzene and furan rings. This colourless liquid is a component of coal tar. Benzofuran is the "parent" of many related compounds with more complex structures. For example, psoralen is a benzofuran derivative that occurs in several plants. Production Benzofuran is extracted from coal tar. It is also obtained by dehydrogenation of 2-ethylphenol. Laboratory methods Benzofurans can be prepared by various methods in the laboratory. Notable examples include: *''O''-alkylation of salicylaldehyde with chloroacetic acid followed by dehydration (cyclication) of the resulting ether and decarboxylation. *Perkin rearrangement, where a coumarin is reacted with a hydroxide: : *Diels–Alder reaction of nitro vinyl furans with various dienophiles: : Diels–Alder reaction yielding a substituted benzofuran, 450px * Cycloisomerization of alkyne ortho-substituted phenols: : Benzofurans via Cycloisomerization, 400px Related compou ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Polymerization
In polymer chemistry, polymerization (American English), or polymerisation (British English), is a process of reacting monomer, monomer molecules together in a chemical reaction to form polymer chains or three-dimensional networks. There are many forms of polymerization and different systems exist to categorize them. In chemical compounds, polymerization can occur via a variety of reaction mechanisms that vary in complexity due to the functional groups present in the reactants and their inherent steric effects. In more straightforward polymerizations, alkenes form polymers through relatively simple free-radical reaction, radical reactions; in contrast, reactions involving substitution at a carbonyl group require more complex synthesis due to the way in which reactants polymerize. Alkanes can also be polymerized, but only with the help of strong acids. As alkenes can polymerize in somewhat straightforward radical reactions, they form useful compounds such as polyethylene and p ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Sodium
Sodium is a chemical element with the symbol Na (from Latin ''natrium'') and atomic number 11. It is a soft, silvery-white, highly reactive metal. Sodium is an alkali metal, being in group 1 of the periodic table. Its only stable isotope is 23Na. The free metal does not occur in nature, and must be prepared from compounds. Sodium is the sixth most abundant element in the Earth's crust and exists in numerous minerals such as feldspars, sodalite, and halite (NaCl). Many salts of sodium are highly water-soluble: sodium ions have been leached by the action of water from the Earth's minerals over eons, and thus sodium and chlorine are the most common dissolved elements by weight in the oceans. Sodium was first isolated by Humphry Davy in 1807 by the electrolysis of sodium hydroxide. Among many other useful sodium compounds, sodium hydroxide (lye) is used in soap manufacture, and sodium chloride (edible salt) is a de-icing agent and a nutrient for animals including h ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Deprotonation
Deprotonation (or dehydronation) is the removal (transfer) of a proton (or hydron, or hydrogen cation), (H+) from a Brønsted–Lowry acid in an acid–base reaction.Henry Jakubowski, Biochemistry Online Chapter 2A3, https://employees.csbsju.edu/hjakubowski/classes/ch331/protstructure/PS_2A3_AA_Charges.html, accessed 12/2/2020 The species formed is the conjugate base of that acid. The complementary process, when a proton is added (transferred) to a Brønsted–Lowry base, is protonation (or hydronation). The species formed is the conjugate acid of that base. A species that can either accept or donate a proton is referred to as amphiprotic. An example is the H2O (water) molecule, which can gain a proton to form the hydronium ion, H3O+, or lose a proton, leaving the hydroxide ion, OH−. The relative ability of a molecule to give up a proton is measured by its p''K''a value. A low p''K''a value indicates that the compound is acidic and will easily give up its proton to a base ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Distillation
Distillation, or classical distillation, is the process of separation process, separating the components or substances from a liquid mixture by using selective boiling and condensation, usually inside an apparatus known as a still. Dry distillation is the heating of solid materials to produce gaseous products (which may condense into liquids or solids); this may involve chemical changes such as destructive distillation or Cracking (chemistry), cracking. Distillation may result in essentially complete separation (resulting in nearly pure components), or it may be a partial separation that increases the concentration of selected components; in either case, the process exploits differences in the relative volatility of the mixture's components. In Chemical industry, industrial applications, distillation is a unit operation of practically universal importance, but is a physical separation process, not a chemical reaction. An installation used for distillation, especially of distilled ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
International Union Of Pure And Applied Chemistry
The International Union of Pure and Applied Chemistry (IUPAC ) is an international federation of National Adhering Organizations working for the advancement of the chemical sciences, especially by developing nomenclature and terminology. It is a member of the International Science Council (ISC). IUPAC is registered in Zürich, Switzerland, and the administrative office, known as the "IUPAC Secretariat", is in Research Triangle Park, North Carolina, United States. This administrative office is headed by IUPAC's executive director, currently Lynn Soby. IUPAC was established in 1919 as the successor of the International Congress of Applied Chemistry for the advancement of chemistry. Its members, the National Adhering Organizations, can be national chemistry societies, national academies of sciences, or other bodies representing chemists. There are fifty-four National Adhering Organizations and three Associate National Adhering Organizations. IUPAC's Inter-divisional Committee on ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Fraction (chemistry)
A fraction in chemistry is a quantity collected from a batch of a substance in a fractionating separation process. In such a process, a mixture is separated into fractions, which have compositions that vary according to a gradient. A fraction can be defined as a group of chemicals that have similar boiling points. A common fractionating process is fractional distillation. It is used to produce liquor and various hydrocarbon fuels, such as gasoline, kerosene and diesel. A fraction is the product of a fractionating column, a vast chamber designed to separate different substances (such as crude oil) based on their boiling point. Fraction could also refer to a description of the composition of a mixture, e.g. mass fraction or mole fraction In chemistry, the mole fraction or molar fraction (''xi'' or ) is defined as unit of the amount of a constituent (expressed in moles), ''ni'', divided by the total amount of all constituents in a mixture (also expressed in moles), ''n''tot. This ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |