|
Hexamethylcyclotrisilazane
Hexamethylcyclotrisilazane is a chemical compound with formula or . Its molecule consists of a six-member ring of three silicon atoms alternating with three nitrogen atoms, with two methyl groups bonded to each silicon and one hydrogen atom bonded to each nitrogen. It can be described as a derivative of the hypothetical compound cyclotrisilazane , or as a cyclic trimer of hypothetical dimethylsilazane . The compound is a clear colorless liquid at room temperature. The compound has been extensively studied because of its applications in the semiconductor industry, as a precursor for the deposition of fils of silicon nitride and silicon carbonitride and as an additive in photoresist formulations. It has also been proposed as an additive to silica for liquid chromatography. Other names for the compound are 2,2,4,4,6,6-hexamethyl-1,3,5,2,4,6-triazatrisilinane (IUPAC), 1,1,3,3,5,5-hexamethyl-2,4,6,1,3,5-triazatrisilinane, and 2,4,4,6,6-hexamethyl-1,3,5-triaza-2,4,6-trisilacyc ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
2,2,4,4,6,6-hexamethyl-1,3,5-trithiane
Trithioacetone (2,2,4,4,6,6-hexamethyl-1,3,5-trithiane) is an organic chemical with formula . Its covalent structure is , that is, a six-membered ring of alternating carbon and sulfur atoms, with two methyl groups attached to each carbon. It can be viewed as a derivative of 1,3,5-trithiane, with methyl-group substituents for all of the hydrogen atoms in that parent structure. The compound Trithioacetone is a stable cyclic trimer of thioacetone (propane-2-thione), which by itself is an unstable compound. In contrast, the analogous trioxane compound, 2,2,4,4,6,6-hexamethyl-1,3,5-trioxane ( Triacetone), with oxygen atoms in place of the sulfur atoms, seems to be unstable, while its corresponding monomer acetone (2-propanone) is stable. Synthesis Trithioacetone was first made in 1889 by Baumann and Fromm, by reaction of hydrogen sulfide with acetone. In the presence of an acidified catalyst at 25 °C, one obtains a product that is 60–70% trithioacetone, 30–40% of 2,2-prop ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Chemical Compound
A chemical compound is a chemical substance composed of many identical molecules (or molecular entities) containing atoms from more than one chemical element held together by chemical bonds. A molecule consisting of atoms of only one element is therefore not a compound. A compound can be transformed into a different substance by a chemical reaction, which may involve interactions with other substances. In this process, bonds between atoms may be broken and/or new bonds formed. There are four major types of compounds, distinguished by how the constituent atoms are bonded together. Molecular compounds are held together by covalent bonds; ionic compounds are held together by ionic bonds; intermetallic compounds are held together by metallic bonds; coordination complexes are held together by coordinate covalent bonds. Non-stoichiometric compounds form a disputed marginal case. A chemical formula specifies the number of atoms of each element in a compound molecule, using the s ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Photoresist
A photoresist (also known simply as a resist) is a light-sensitive material used in several processes, such as photolithography and photoengraving, to form a patterned coating on a surface. This process is crucial in the electronic industry. The process begins by coating a substrate with a light-sensitive organic material. A patterned mask is then applied to the surface to block light, so that only unmasked regions of the material will be exposed to light. A solvent, called a developer, is then applied to the surface. In the case of a positive photoresist, the photo-sensitive material is degraded by light and the developer will dissolve away the regions that were exposed to light, leaving behind a coating where the mask was placed. In the case of a negative photoresist, the photosensitive material is strengthened (either polymerized or cross-linked) by light, and the developer will dissolve away only the regions that were not exposed to light, leaving behind a coating in areas w ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Tetramer
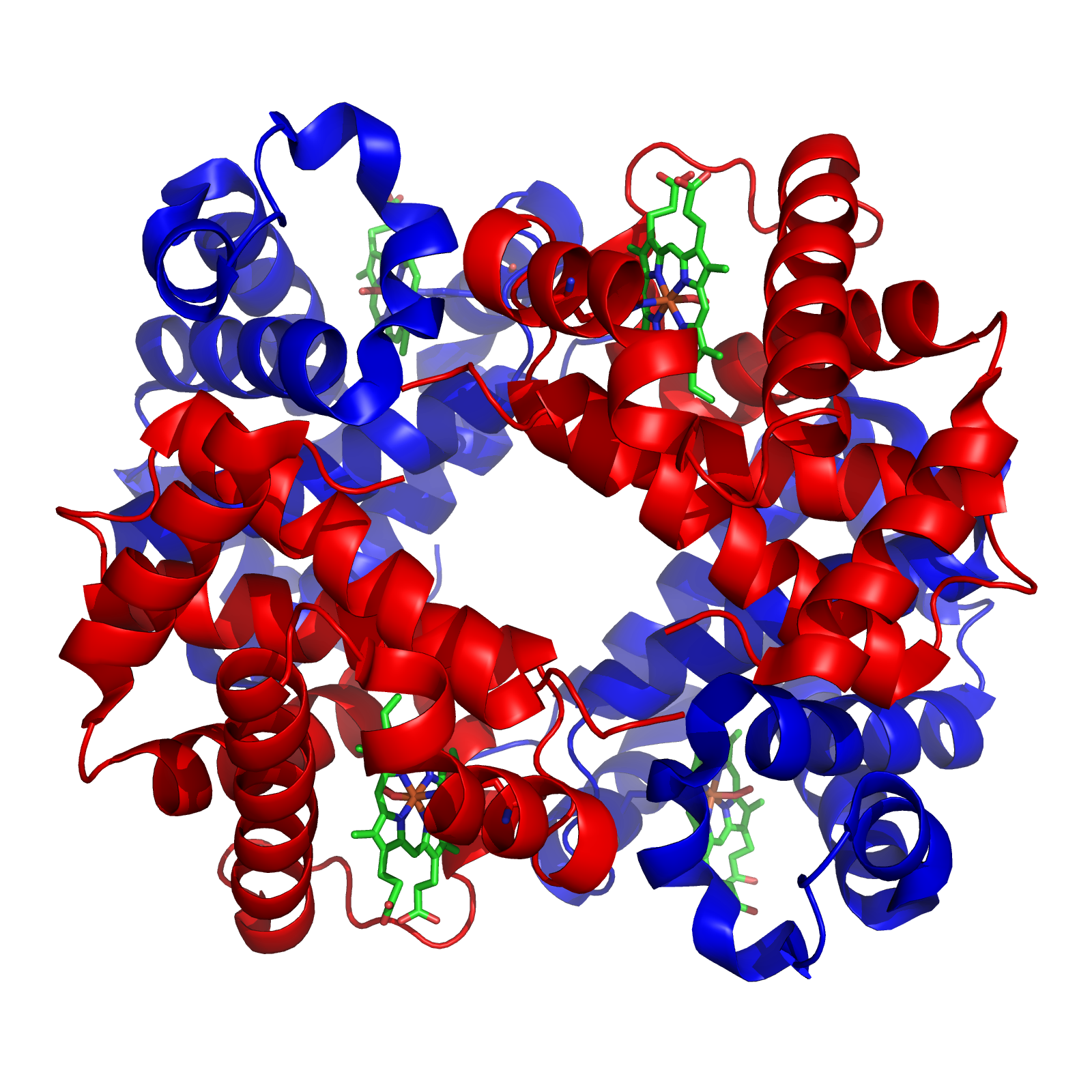
A tetramer () (''tetra-'', "four" + '' -mer'', "parts") is an oligomer formed from four monomers or subunits. The associated property is called ''tetramery''. An example from inorganic chemistry is titanium methoxide with the empirical formula Ti(OCH3)4, which is tetrameric in solid state and has the molecular formula Ti4(OCH3)16. An example from organic chemistry is kobophenol A, a substance that is formed by combining four molecules of resveratrol. In biochemistry, it similarly refers to a biomolecule formed of four units, that are the same (homotetramer), i.e. as in Concanavalin A or different (heterotetramer), i.e. as in hemoglobin. Hemoglobin has 4 similar sub-units while immunoglobulins have 2 very different sub-units. The different sub-units may have each their own activity, such as binding biotin in avidin tetramers, or have a common biological property, such as the allosteric binding of oxygen in hemoglobin. See also * Cluster chemistry; atomic and molecular clusters ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Benzene
Benzene is an organic chemical compound with the molecular formula C6H6. The benzene molecule is composed of six carbon atoms joined in a planar ring with one hydrogen atom attached to each. Because it contains only carbon and hydrogen atoms, benzene is classed as a hydrocarbon. Benzene is a natural constituent of petroleum and is one of the elementary petrochemicals. Due to the cyclic continuous pi bonds between the carbon atoms, benzene is classed as an aromatic hydrocarbon. Benzene is a colorless and highly flammable liquid with a sweet smell, and is partially responsible for the aroma of gasoline. It is used primarily as a precursor to the manufacture of chemicals with more complex structure, such as ethylbenzene and cumene, of which billions of kilograms are produced annually. Although benzene is a major industrial chemical, it finds limited use in consumer items because of its toxicity. History Discovery The word "''benzene''" derives from "''gum benzoin''" (benzoin res ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Ammonia
Ammonia is an inorganic compound of nitrogen and hydrogen with the formula . A stable binary hydride, and the simplest pnictogen hydride, ammonia is a colourless gas with a distinct pungent smell. Biologically, it is a common nitrogenous waste, particularly among aquatic organisms, and it contributes significantly to the nutritional needs of terrestrial organisms by serving as a precursor to 45% of the world's food and fertilizers. Around 70% of ammonia is used to make fertilisers in various forms and composition, such as urea and Diammonium phosphate. Ammonia in pure form is also applied directly into the soil. Ammonia, either directly or indirectly, is also a building block for the synthesis of many pharmaceutical products and is used in many commercial cleaning products. It is mainly collected by downward displacement of both air and water. Although common in nature—both terrestrially and in the outer planets of the Solar System—and in wide use, ammonia is both caust ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Dimethyldichlorosilane
Dimethyldichlorosilane is a tetrahedral, organosilicon compound with the formula Si(CH3)2Cl2. At room temperature it is a colorless liquid that readily reacts with water to form both linear and cyclic Si-O chains. Dimethyldichlorosilane is made on an industrial scale as the principal precursor to dimethylsilicone and polysilane compounds. History The first organosilicon compounds were reported in 1863 by Charles Friedel and James Crafts who synthesized tetraethylsilane from diethylzinc and silicon tetrachloride.Silicon: Organosilicon Chemistry. Encyclopedia of Inorganic Chemistry Online, 2nd ed.; Wiley: New Jersey, 2005. However, major progress in organosilicon chemistry did not occur until Frederick Kipping and his students began experimenting with diorganodichlorosilanes (R2SiCl2) that were prepared by reacting silicon tetrachloride with Grignard reagents. Unfortunately, this method suffered from many experimental problems. In the 1930s, the demand for silicones increased ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Angstrom
The angstromEntry "angstrom" in the Oxford online dictionary. Retrieved on 2019-03-02 from https://en.oxforddictionaries.com/definition/angstrom.Entry "angstrom" in the Merriam-Webster online dictionary. Retrieved on 2019-03-02 from https://www.merriam-webster.com/dictionary/angstrom. (, ; , ) or ångström is a metric unit of length equal to m; that is, one ten-billionth ( US) of a metre, a hundred-millionth of a centimetre,Entry "angstrom" in the Oxford English Dictionary, 2nd edition (1986). Retrieved on 2021-11-22 from https://www.oed.com/oed2/00008552. 0.1 nanometre, or 100 picometres. Its symbol is Å, a letter of the Swedish alphabet. The unit is named after the Swedish physicist Anders Jonas Ångström (1814–1874). The angstrom is often used in the natural sciences and technology to express sizes of atoms, molecules, microscopic biological structures, and lengths of chemical bonds, arrangement of atoms in crystals,Arturas Vailionis (2015):Geometry of Crystals Lect ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
IUPAC
The International Union of Pure and Applied Chemistry (IUPAC ) is an international federation of National Adhering Organizations working for the advancement of the chemical sciences, especially by developing nomenclature and terminology. It is a member of the International Science Council (ISC). IUPAC is registered in Zürich, Switzerland, and the administrative office, known as the "IUPAC Secretariat", is in Research Triangle Park, North Carolina, United States. This administrative office is headed by IUPAC's executive director, currently Lynn Soby. IUPAC was established in 1919 as the successor of the International Congress of Applied Chemistry for the advancement of chemistry. Its members, the National Adhering Organizations, can be national chemistry societies, national academies of sciences, or other bodies representing chemists. There are fifty-four National Adhering Organizations and three Associate National Adhering Organizations. IUPAC's Inter-divisional Committee on ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Liquid Chromatography
In chemical analysis, chromatography is a laboratory technique for the Separation process, separation of a mixture into its components. The mixture is dissolved in a fluid solvent (gas or liquid) called the ''mobile phase'', which carries it through a system (a column, a capillary tube, a plate, or a sheet) on which a material called the ''stationary phase'' is fixed. Because the different constituents of the mixture tend to have different affinities for the stationary phase and are retained for different lengths of time depending on their interactions with its surface sites, the constituents travel at different apparent velocities in the mobile fluid, causing them to separate. The separation is based on the differential partitioning between the mobile and the stationary phases. Subtle differences in a compound's partition coefficient result in differential retention on the stationary phase and thus affect the separation. Chromatography may be preparative or analytical. The pu ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Silica
Silicon dioxide, also known as silica, is an oxide of silicon with the chemical formula , most commonly found in nature as quartz and in various living organisms. In many parts of the world, silica is the major constituent of sand. Silica is one of the most complex and most abundant families of materials, existing as a compound of several minerals and as a synthetic product. Notable examples include fused quartz, fumed silica, silica gel, opal and aerogels. It is used in structural materials, microelectronics (as an Insulator (electricity), electrical insulator), and as components in the food and pharmaceutical industries. Structure In the majority of silicates, the silicon atom shows tetrahedral coordination geometry, tetrahedral coordination, with four oxygen atoms surrounding a central Si atomsee 3-D Unit Cell. Thus, SiO2 forms 3-dimensional network solids in which each silicon atom is covalently bonded in a tetrahedral manner to 4 oxygen atoms. In contrast, CO2 is a linear ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |

2.png)
-3D-balls.png)
_【_Pictures_taken_in_Japan_】_(cropped).jpg)