|
Hexafluoropropylenoxide
Hexafluoropropylene oxide (HFPO) is an intermediate used in industrial organofluorine chemistry; specifically it is a monomer for fluoropolymers. This colourless gas is the epoxide of hexafluoropropylene, that is fluorinated analog of propylene oxide, HFPO is produced by DuPont and 3M and as a precursor to the lubricant Krytox and related materials. It is generated by oxidation of perfluoropropylene, e.g. with oxygen as well as other oxidants. Reactivity Fluoride catalyzes the formation of the perfluorinated polyethers such as Krytox. The initial step entails nucleophilic attack at the middle carbon to give the perfluoropropoxide anion, which in turn attacks another monomer. This process generates a polymer terminated by an acyl fluoride, which is hydrolyzed to the carboxylic acid which is decarboxylated with fluorine. The net polymerization reaction can be represented as: :n+2 CF3CFCF2O → CF3CF2CF2O(CF(CF3)CF2O)nCF2CF3 + CO Upon heating above 150 °C, HFPO decomposes ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Organofluorine Chemistry
Organofluorine chemistry describes the chemistry of the organofluorines, organic compounds that contain the carbon–fluorine bond. Organofluorine compounds find diverse applications ranging from Lipophobicity, oil and hydrophobe, water repellents to pharmaceuticals, refrigerants, and reagents in catalysis. In addition to these applications, some organofluorine compounds are pollutants because of their contributions to ozone depletion, global warming, bioaccumulation, and toxicity. The area of organofluorine chemistry often requires special techniques associated with the handling of fluorinating agents. The carbon–fluorine bond Fluorine has several distinctive differences from all other substituents encountered in organic molecules. As a result, the physical and chemical properties of organofluorines can be distinctive in comparison to other organohalogens. # The carbon–fluorine bond is one of the strongest in organic chemistry (an average bond energy around 480 kJ/molKirsch ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Carboxylic Acid
In organic chemistry, a carboxylic acid is an organic acid that contains a carboxyl group () attached to an R-group. The general formula of a carboxylic acid is or , with R referring to the alkyl, alkenyl, aryl, or other group. Carboxylic acids occur widely. Important examples include the amino acids and fatty acids. Deprotonation of a carboxylic acid gives a carboxylate anion. Examples and nomenclature Carboxylic acids are commonly identified by their trivial names. They at oftentimes have the suffix ''-ic acid''. IUPAC-recommended names also exist; in this system, carboxylic acids have an ''-oic acid'' suffix. For example, butyric acid (C3H7CO2H) is butanoic acid by IUPAC guidelines. For nomenclature of complex molecules containing a carboxylic acid, the carboxyl can be considered position one of the parent chain even if there are other substituents, such as 3-chloropropanoic acid. Alternately, it can be named as a "carboxy" or "carboxylic acid" substituent on another ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Epoxides
In organic chemistry, an epoxide is a cyclic ether () with a three-atom ring. This ring approximates an equilateral triangle, which makes it strained, and hence highly reactive, more so than other ethers. They are produced on a large scale for many applications. In general, low molecular weight epoxides are colourless and nonpolar, and often volatile. Nomenclature A compound containing the epoxide functional group can be called an epoxy, epoxide, oxirane, and ethoxyline. Simple epoxides are often referred to as oxides. Thus, the epoxide of ethylene (C2H4) is ethylene oxide (C2H4O). Many compounds have trivial names; for instance, ethylene oxide is called "oxirane". Some names emphasize the presence of the epoxide functional group, as in the compound ''1,2-epoxyheptane'', which can also be called ''1,2-heptene oxide''. A polymer formed from epoxide precursors is called an ''epoxy'', but such materials do not contain epoxide groups (or contain only a few residual epoxy grou ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
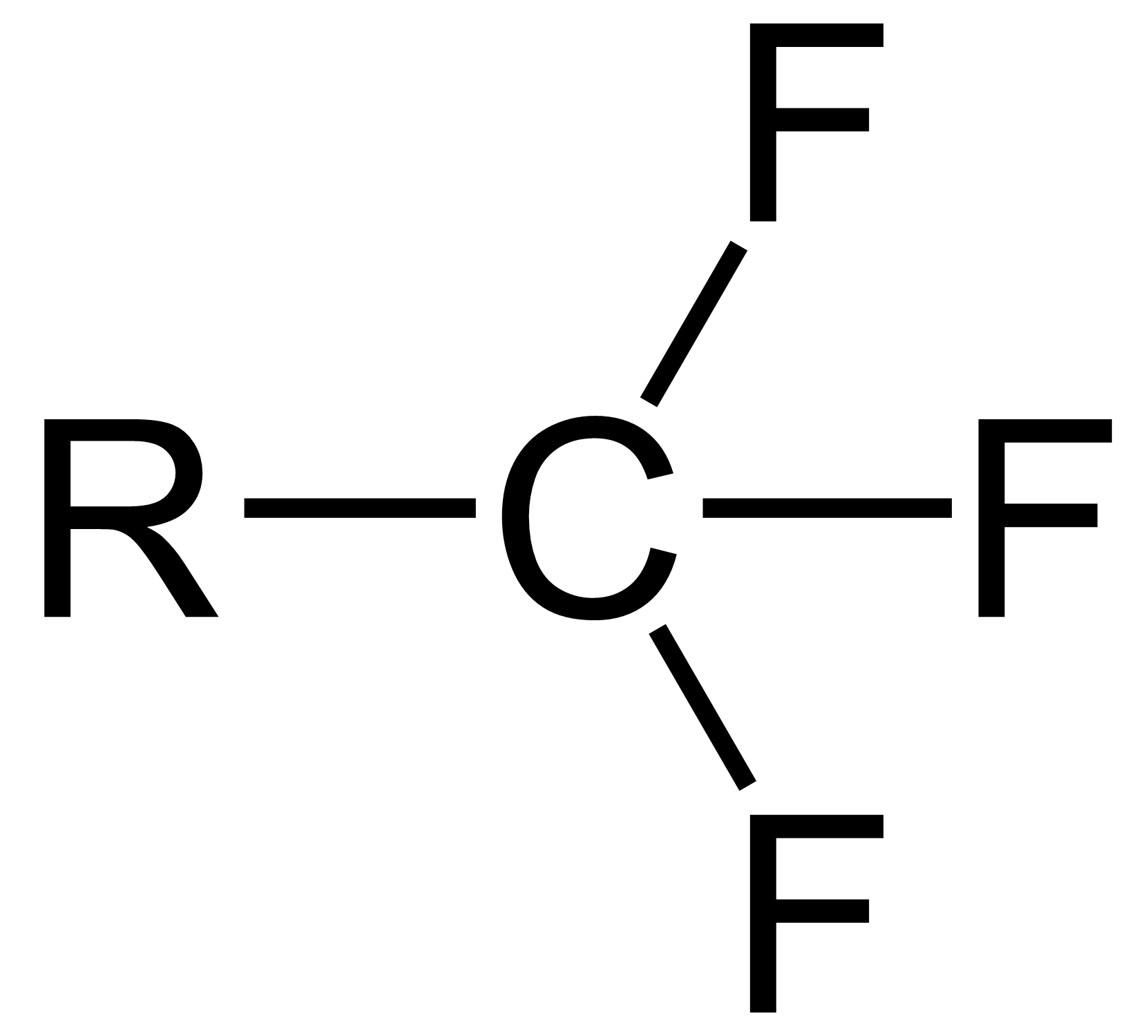
Trifluoromethyl Compounds
The trifluoromethyl group is a functional group that has the formula -CF3. The naming of is group is derived from the methyl group (which has the formula -CH3), by replacing each hydrogen atom by a fluorine atom. Some common examples are trifluoromethane H–, 1,1,1-trifluoroethane –, and hexafluoroacetone –CO–. Compounds with this group are a subclass of the organofluorines. Properties The trifluoromethyl group has a significant electronegativity that is often described as being intermediate between the electronegativities of fluorine and chlorine. For this reason, trifluoromethyl-substituted compounds are often strong acids, such as trifluoromethanesulfonic acid and trifluoroacetic acid. Conversely, the trifluoromethyl group lowers the basicity of compounds like trifluoroethanol. Uses The trifluoromethyl group occurs in certain pharmaceuticals, drugs, and abiotically synthesized natural fluorocarbon based compounds. The medicinal use of the trifloromethyl group dates from ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Hexafluoroacetone
Hexafluoroacetone (HFA) is a chemical compound with the formula (CF3)2CO. It is structurally similar to acetone; however, its reactivity is markedly different. It a colourless, hygroscopic, nonflammable, highly reactive gas characterized by a musty odour. The most common form of this substance is hexafluoroacetone sesquihydrate (1.5 H2O), which is a hemihydrate of hexafluoropropane-2,2-diol , a geminal diol. Synthesis The industrial route to HFA involves treatment of hexachloroacetone with HF: :(CCl3)2CO + 6 HF → (CF3)2CO + 6 HCl Hexafluoropropylene oxide rearranges to give HFA. In the laboratory, HFA can be prepared in a two step process from perfluoropropene. In the first step KF catalyzes the reaction of the alkene with elemental sulfur to give the 1,3-dithietane CF3)2CSsub>2. This species is then oxidized by iodate to give (CF3)2CO. Uses Hexafluoroacetone is used in the production of hexafluoroisopropanol: :(CF3)2CO + H2 → (CF3)2CHOH It is also used as a precursor ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Lewis Acid
A Lewis acid (named for the American physical chemist Gilbert N. Lewis) is a chemical species that contains an empty orbital which is capable of accepting an electron pair from a Lewis base to form a Lewis adduct. A Lewis base, then, is any species that has a filled orbital containing an electron pair which is not involved in bonding but may form a dative bond with a Lewis acid to form a Lewis adduct. For example, NH3 is a Lewis base, because it can donate its lone pair of electrons. Trimethylborane (Me3B) is a Lewis acid as it is capable of accepting a lone pair. In a Lewis adduct, the Lewis acid and base share an electron pair furnished by the Lewis base, forming a dative bond. In the context of a specific chemical reaction between NH3 and Me3B, a lone pair from NH3 will form a dative bond with the empty orbital of Me3B to form an adduct NH3•BMe3. The terminology refers to the contributions of Gilbert N. Lewis. From p. 142: "We are inclined to think of substances as po ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Tetrafluoroethylene
Tetrafluoroethylene (TFE) is a fluorocarbon with the chemical formula C2 F4. It is the simplest perfluorinated alkene. This gaseous species is used primarily in the industrial preparation of fluoropolymers. Properties Tetrafluoroethylene is a colorless, odorless gas. Like all unsaturated fluorocarbons, it is susceptible to nucleophilic attack. It is unstable towards decomposition to carbon and carbon tetrafluoride () and prone to form explosive peroxides in contact with air. Industrial use Polymerization of tetrafluoroethylene produces polytetrafluoroethylene (PTFE) polymers such as Teflon and Fluon. PTFE is one of the two fluorocarbon resins composed wholly of fluorine and carbon. The other resin composed purely of carbon and fluorine is the copolymer of TFE with typically 6–9% hexafluoropropene (HFP), which is known as FEP (fluorinated ethylene propylene copolymer). TFE is also used in the preparation of numerous copolymers that also include hydrogen and/or oxygen, includ ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Difluorocarbene
Difluorocarbene is the chemical compound with formula CF2. It has a short half-life, 0.5 and 20 ms, in solution and in the gas phase, respectively.Douglas A Jean Osteraas "Difluorocarbene Modification of Polymer and Fiber Surfaces," ''Journal of Applied Polymer''1969, volume 13, 1523-1535. Although highly reactive, difluorocarbene is an intermediate in the production of tetrafluoroethylene, which is produced on an industrial scale as the precursor to Teflon ( PTFE). Bonding in difluorocarbene In general, carbenes exist in either singlet or triplet states, which are often quite close in energy. Singlet carbenes have spin-paired electrons and a higher energy empty 2p orbital. In a triplet carbene, one electron occupies the hybrid orbital and the other is promoted to the 2p orbital.Jones. Maitland.''Organic Chemistry'', 3rd ed, W. W. Norton, 2005, 460-465. . For most carbenes, the triplet state is more stable than the corresponding singlet. In the case of fluorinated carbenes ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Acyl Fluoride
In organic chemistry, an acyl halide (also known as an acid halide) is a chemical compound derived from an oxoacid by replacing a hydroxyl group () with a halide group (, where X is a halogen). If the acid is a carboxylic acid (), the compound contains a functional group, which consists of a carbonyl group () singly bonded to a halogen atom. The general formula for such an acyl halide can be written RCOX, where R may be, for example, an alkyl group, CO is the carbonyl group, and X represents the halide, such as chloride. Acyl chlorides are the most commonly encountered acyl halides, but acetyl iodide is the one produced (transiently) on the largest scale. Billions of kilograms are generated annually in the production of acetic acid. Preparation Aliphatic acyl halides On an industrial scale, the reaction of acetic anhydride with hydrogen chloride produces a mixture of acetyl chloride and acetic acid: :(CH3CO)2O + HCl -> CH3COCl + CH3CO2H Common syntheses of acyl chlorides als ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Fluoropolymer
A fluoropolymer is a fluorocarbon-based polymer with multiple carbon–fluorine bonds. It is characterized by a high resistance to solvents, acids, and bases. The best known fluoropolymer is polytetrafluoroethylene under the brand name "Teflon," trademarked by the DuPont Company. History In 1938, polytetrafluoroethylene (DuPont brand name Teflon) was discovered by accident by a recently hired DuPont Ph.D., Roy J. Plunkett. While working with tetrafluoroethylene gas to develop refrigerants, he noticed that a previously pressurized cylinder had no pressure remaining. In dissecting the cylinder, he found a mass of white solid in a quantity similar to that of the tetrafluoroethylene gas. It was determined that this material was a new-to-the-world polymer. Tests showed the substance was resistant to corrosion from most acids, bases and solvents and had better high temperature stability than any other plastic. By early 1941, a crash program was making substantial quantities of P ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Nucleophilic Attack
In chemistry, a nucleophile is a chemical species that forms bonds by donating an electron pair. All molecules and ions with a free pair of electrons or at least one pi bond can act as nucleophiles. Because nucleophiles donate electrons, they are Lewis bases. ''Nucleophilic'' describes the affinity of a nucleophile to bond with positively charged atomic nuclei. Nucleophilicity, sometimes referred to as nucleophile strength, refers to a substance's nucleophilic character and is often used to compare the affinity of atoms. Neutral nucleophilic reactions with solvents such as alcohols and water are named solvolysis. Nucleophiles may take part in nucleophilic substitution, whereby a nucleophile becomes attracted to a full or partial positive charge, and nucleophilic addition. Nucleophilicity is closely related to basicity. History The terms ''nucleophile'' and ''electrophile'' were introduced by Christopher Kelk Ingold in 1933, replacing the terms ''anionoid'' and ''cationoid'' ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Krytox
Krytox is a registered trademark of The Chemours Company. It refers to a group of colourless synthetic lubricants (oils and greases) with a variety of applications. Invented by researchers at DuPont, Krytox oils are fluorocarbon ether polymers of polyhexafluoropropylene oxide, with a chemical formula: F−(CF(CF3)−CF2−O)''n''−CF2CF3, where the degree of polymerization, n, generally lies within the range of 10 to 60. DuPont Krytox Performance Lubricants Product Overview, available onlinhere These compounds are collectively known by many names including perfluoropolyether (PFPE), perfluoroalkylether (PFAE) and perfluoropolyalkylether (PFPAE). A unique identifier is their CAS registry number, 60164-51-4. In addition to PFPE, Krytox grease also contains telomers of PTFE and in fact was designed as a liquid or grease form of PTFE. It is thermally stable, nonflammable (even in liquid oxygen), and insoluble in water, acids, bases, and most organic solvents. It is nonvolatile and ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |