|
Tetrafluoroethylene
Tetrafluoroethylene (TFE) is a fluorocarbon with the chemical formula C2 F4. It is the simplest perfluorinated alkene. This gaseous species is used primarily in the industrial preparation of fluoropolymers. Properties Tetrafluoroethylene is a colorless, odorless gas. Like all unsaturated fluorocarbons, it is susceptible to nucleophilic attack. It is unstable towards decomposition to carbon and carbon tetrafluoride () and prone to form explosive peroxides in contact with air. Industrial use Polymerization of tetrafluoroethylene produces polytetrafluoroethylene (PTFE) polymers such as Teflon and Fluon. PTFE is one of the two fluorocarbon resins composed wholly of fluorine and carbon. The other resin composed purely of carbon and fluorine is the copolymer of TFE with typically 6–9% hexafluoropropene (HFP), which is known as FEP (fluorinated ethylene propylene copolymer). TFE is also used in the preparation of numerous copolymers that also include hydrogen and/or oxygen, i ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Fluorocarbon
Fluorocarbons are chemical compounds with carbon-fluorine bonds. Compounds that contain many C-F bonds often has distinctive properties, e.g., enhanced stability, volatility, and hydrophobicity. Fluorocarbons and their derivatives are commercial polymers, refrigerants, drugs, and anesthetics. Nomenclature Perfluorocarbons or PFCs, are organofluorine compounds with the formula CxFy, i.e., they contain only carbon and fluorine. The terminology is not strictly followed and many fluorine-containing organic compounds are called fluorocarbons. Compounds with the prefix perfluoro- are hydrocarbons, including those with heteroatoms, wherein all C-H bonds have been replaced by C-F bonds. Fluorocarbons includes perfluoroalkanes, fluoroalkenes, fluoroalkynes, and perfluoroaromatic compounds. Perfluoroalkanes Chemical properties Perfluoroalkanes are very stable because of the strength of the carbon–fluorine bond, one of the strongest in organic chemistry. Its strength is ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Teflon
Polytetrafluoroethylene (PTFE) is a synthetic fluoropolymer of tetrafluoroethylene that has numerous applications. It is one of the best-known and widely applied PFAS. The commonly known brand name of PTFE-based composition is Teflon by Chemours, a spin-off from DuPont, which originally discovered the compound in 1938. Polytetrafluoroethylene is a fluorocarbon solid, as it is a high- molecular-weight polymer consisting wholly of carbon and fluorine. PTFE is hydrophobic: neither water nor water-containing substances wet PTFE, as fluorocarbons exhibit only small London dispersion forces due to the low electric polarizability of fluorine. PTFE has one of the lowest coefficients of friction of any solid. Polytetrafluoroethylene is used as a non-stick coating for pans and other cookware. It is non-reactive, partly because of the strength of carbon–fluorine bonds, so it is often used in containers and pipework for reactive and corrosive chemicals. Where used as a lubrican ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Fluoropolymer
A fluoropolymer is a fluorocarbon-based polymer with multiple carbon–fluorine bonds. It is characterized by a high resistance to solvents, acids, and bases. The best known fluoropolymer is polytetrafluoroethylene under the brand name "Teflon," trademarked by the DuPont Company. History In 1938, polytetrafluoroethylene ( DuPont brand name Teflon) was discovered by accident by a recently hired DuPont Ph.D., Roy J. Plunkett. While working with tetrafluoroethylene gas to develop refrigerants, he noticed that a previously pressurized cylinder had no pressure remaining. In dissecting the cylinder, he found a mass of white solid in a quantity similar to that of the tetrafluoroethylene gas. It was determined that this material was a new-to-the-world polymer. Tests showed the substance was resistant to corrosion from most acids, bases and solvents and had better high temperature stability than any other plastic. By early 1941, a crash program was making substantial quantities of P ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Polytetrafluoroethylene
Polytetrafluoroethylene (PTFE) is a synthetic fluoropolymer of tetrafluoroethylene that has numerous applications. It is one of the best-known and widely applied PFAS. The commonly known brand name of PTFE-based composition is Teflon by Chemours, a spin-off from DuPont, which originally discovered the compound in 1938. Polytetrafluoroethylene is a fluorocarbon solid, as it is a high- molecular-weight polymer consisting wholly of carbon and fluorine. PTFE is hydrophobic: neither water nor water-containing substances wet PTFE, as fluorocarbons exhibit only small London dispersion forces due to the low electric polarizability of fluorine. PTFE has one of the lowest coefficients of friction of any solid. Polytetrafluoroethylene is used as a non-stick coating for pans and other cookware. It is non-reactive, partly because of the strength of carbon–fluorine bonds, so it is often used in containers and pipework for reactive and corrosive chemicals. Where used as a lubri ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Perfluorinated Compound
A perfluorinated compound (PFC) or perfluoro compound is an organofluorine compound containing only carbon-fluorines and C−C bonds, as well as potentially heteroatoms. Perfluorinated compounds have properties that result from the presence of fluorocarbons (containing only C−F and C−C bonds) and any functional group. Common functional groups in PFCs are OH, CO2H, chlorine, O, and SO3H. Electrofluorination is the predominant method of production. Some of these compounds known as perfluoroalkanes can remain in our atmosphere for a long time. They bioaccumulate due to their chemical stability. Because of their potential contribution to climate change, they were regulated under the Kyoto Protocol. Some fluorosurfactants have proven toxic in animal testing while widespread industrial applications continue. Applications Perfluorinated compounds are used ubiquitously: For example, fluorosurfactants are widely used in the production of teflon (PTFE) and related fluorinated poly ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Difluorocarbene
Difluorocarbene is the chemical compound with formula CF2. It has a short half-life, 0.5 and 20 ms, in solution and in the gas phase, respectively.Douglas A Jean Osteraas "Difluorocarbene Modification of Polymer and Fiber Surfaces," ''Journal of Applied Polymer''1969, volume 13, 1523-1535. Although highly reactive, difluorocarbene is an intermediate in the production of tetrafluoroethylene, which is produced on an industrial scale as the precursor to Teflon (PTFE). Bonding in difluorocarbene In general, carbenes exist in either singlet or triplet states, which are often quite close in energy. Singlet carbenes have spin-paired electrons and a higher energy empty 2p orbital. In a triplet carbene, one electron occupies the hybrid orbital and the other is promoted to the 2p orbital.Jones. Maitland.''Organic Chemistry'', 3rd ed, W. W. Norton, 2005, 460-465. . For most carbenes, the triplet state is more stable than the corresponding singlet. In the case of fluorinated carbenes, ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
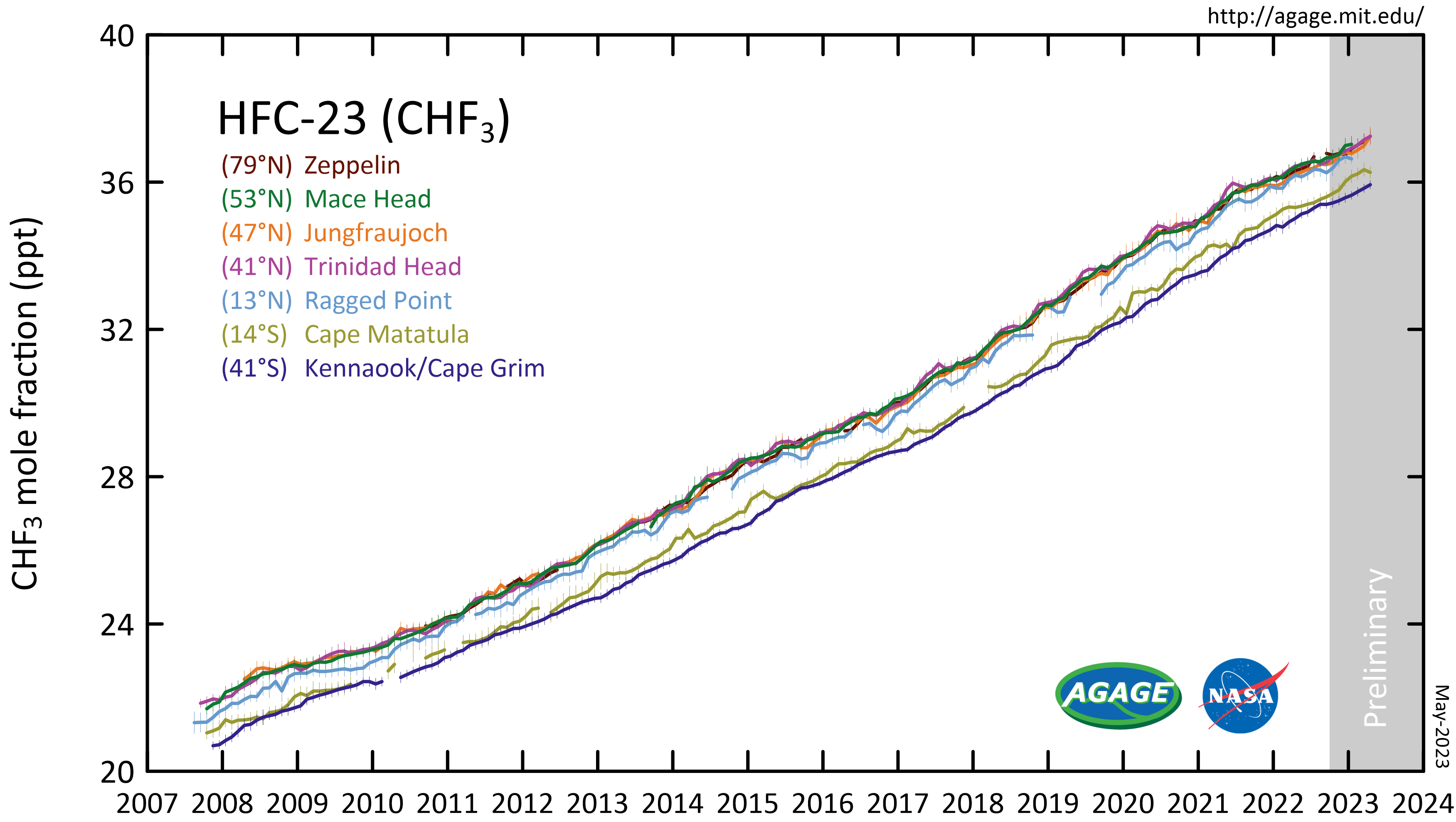
Fluoroform
Trifluoromethane or fluoroform is the chemical compound with the formula CHF3. It is one of the " haloforms", a class of compounds with the formula CHX3 (X = halogen) with C3v symmetry. Fluoroform is used in diverse applications in organic synthesis. It is not an ozone depleter but is a greenhouse gas. Synthesis About 20M kg/y are produced industrially as both a by-product of and precursor to the manufacture of Teflon. It is produced by reaction of chloroform with HF: :CHCl3 + 3 HF → CHF3 + 3 HCl It is also generated biologically in small amounts apparently by decarboxylation of trifluoroacetic acid. Historical Fluoroform was first obtained by Maurice Meslans in the violent reaction of iodoform with dry silver fluoride in 1894. The reaction was improved by Otto Ruff by substitution of silver fluoride by a mixture of mercury fluoride and calcium fluoride. The exchange reaction works with iodoform and bromoform, and the exchange of the first two halogen atom ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Pentafluoropropionic Acid
Perfluoropropionic acid (PFPrA) or pentafluoropropionic acid is the perfluoroalkyl carboxylic acid with the formula CF3CF2CO2H. It is a colorless liquid that is strongly acidic and soluble in water and polar organic solvents. A convenient, safe method for generating tetrafluoroethylene is the pyrolysis of the sodium salt of pentafluoropropionic acid:{{cite journal , doi = 10.1016/j.jfluchem.2016.10.004, title = Preparation of tetrafluoroethylene from the pyrolysis of pentafluoropropionate salts, year = 2017, last1 = Hercules, first1 = Daniel A., last2 = Parrish, first2 = Cameron A., last3 = Sayler, first3 = Todd S., last4 = Tice, first4 = Kevin T., last5 = Williams, first5 = Shane M., last6 = Lowery, first6 = Lauren E., last7 = Brady, first7 = Michael E., last8 = Coward, first8 = Robert B., last9 = Murphy, first9 = Justin A., last10 = Hey, first10 = Trevyn A., last11 = Scavuzzo, first11 = Anthony R., last12 = Rummler, first12 = Lucy M., last13 = Burns, first13 = Emory G., last1 ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Depolymerization
Depolymerization (or depolymerisation) is the process of converting a polymer into a monomer or a mixture of monomers. This process is driven by an increase in entropy. Ceiling temperature The tendency of polymers to depolymerize is indicated by their ceiling temperature. At this temperature, the enthalpy of polymerization matches the entropy gained by converting a large molecule into monomers. Above the ceiling temperature, the rate of depolymerization is greater than the rate of polymerization, which inhibits the formation of the given polymer. Applications Depolymerization is a very common process. Digestion of food involves depolymerization of macromolecules, such as proteins. It is relevant to polymer recycling. Sometimes the depolymerization is well behaved, and clean monomers can be reclaimed and reused for making new plastic. In other cases, such as polyethylene, depolymerization gives a mixture of products. These products are, for polyethylene, ethylene, propylen ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Fused Quartz
Fused quartz, fused silica or quartz glass is a glass consisting of almost pure silica (silicon dioxide, SiO2) in amorphous (non-crystalline) form. This differs from all other commercial glasses in which other ingredients are added which change the glasses' optical and physical properties, such as lowering the melt temperature. Fused quartz, therefore, has high working and melting temperatures, making it less desirable for most common applications. The terms fused quartz and fused silica are used interchangeably, but can refer to different manufacturing techniques, as noted below, resulting in different trace impurities. However fused quartz, being in the glassy state, has quite different physical properties compared to crystalline quartz. Due to its physical properties it finds specialty uses in semiconductor fabrication and laboratory equipment, for instance. Compared to other common glasses, the optical transmission of pure silica extends well into the ultraviolet and infr ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Cracking (chemistry)
In petrochemistry, petroleum geology and organic chemistry, cracking is the process whereby complex organic molecules such as kerogens or long-chain hydrocarbons are broken down into simpler molecules such as light hydrocarbons, by the breaking of carbon-carbon bonds in the precursors. The rate of cracking and the end products are strongly dependent on the temperature and presence of catalysts. Cracking is the breakdown of a large alkane into smaller, more useful alkenes. Simply put, hydrocarbon cracking is the process of breaking a long chain of hydrocarbons into short ones. This process requires high temperatures. More loosely, outside the field of petroleum chemistry, the term "cracking" is used to describe any type of splitting of molecules under the influence of heat, catalysts and solvents, such as in processes of destructive distillation or pyrolysis. Fluid catalytic cracking produces a high yield of petrol and LPG, while hydrocracking is a major source of jet fuel ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Chlorodifluoromethane
Chlorodifluoromethane or difluoromonochloromethane is a hydrochlorofluorocarbon (HCFC). This colorless gas is better known as HCFC-22, or R-22, or . It was commonly used as a propellant and refrigerant. These applications were phased out under the Montreal Protocol in developed countries in 2020 due to the compound's ozone depletion potential (ODP) and high global warming potential (GWP), and in developing countries this process will be completed by 2030. R-22 is a versatile intermediate in industrial organofluorine chemistry, e.g. as a precursor to tetrafluoroethylene. Production and current applications Worldwide production of R-22 in 2008 was about 800Gg per year, up from about 450Gg per year in 1998, with most production in developing countries. R-22 use is being phased out in developing countries, where it is largely used for air conditioning applications. Air conditioning sales are growing 20% annually in India and China. R-22 is prepared from chloroform: :HCCl3 + 2 HF ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |