|
Helium 3
Helium-3 (3He see also helion) is a light, stable isotope of helium with two protons and one neutron (the most common isotope, helium-4, having two protons and two neutrons in contrast). Other than protium (ordinary hydrogen), helium-3 is the only stable isotope of any element with more protons than neutrons. Helium-3 was discovered in 1939. Helium-3 occurs as a primordial nuclide, escaping from Earth's crust into its atmosphere and into outer space over millions of years. Helium-3 is also thought to be a natural nucleogenic and cosmogenic nuclide, one produced when lithium is bombarded by natural neutrons, which can be released by spontaneous fission and by nuclear reactions with cosmic rays. Some of the helium-3 found in the terrestrial atmosphere is also an artifact of atmospheric and underwater nuclear weapons testing. Much speculation has been made over the possibility of helium-3 as a future energy source. Unlike most nuclear fusion reactions, the fusion of helium-3 at ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Beta Decay
In nuclear physics, beta decay (β-decay) is a type of radioactive decay in which a beta particle (fast energetic electron or positron) is emitted from an atomic nucleus, transforming the original nuclide to an isobar of that nuclide. For example, beta decay of a neutron transforms it into a proton by the emission of an electron accompanied by an antineutrino; or, conversely a proton is converted into a neutron by the emission of a positron with a neutrino in so-called ''positron emission''. Neither the beta particle nor its associated (anti-)neutrino exist within the nucleus prior to beta decay, but are created in the decay process. By this process, unstable atoms obtain a more stable ratio of protons to neutrons. The probability of a nuclide decaying due to beta and other forms of decay is determined by its nuclear binding energy. The binding energies of all existing nuclides form what is called the nuclear band or valley of stability. For either electron or positron em ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Spontaneous Fission
Spontaneous fission (SF) is a form of radioactive decay that is found only in very heavy chemical elements. The nuclear binding energy of the elements reaches its maximum at an atomic mass number of about 56 (e.g., iron-56); spontaneous breakdown into smaller nuclei and a few isolated nuclear particles becomes possible at greater atomic mass numbers. History By 1908, physicists understood that alpha decay involved ejection of helium nuclei from a decaying atom. Like cluster decay, alpha decay is not typically categorized as a process of fission. The first nuclear fission process discovered was fission induced by neutrons. Because cosmic rays produce some neutrons, it was difficult to distinguish between induced and spontaneous events. Cosmic rays can be reliably shielded by a thick layer of rock or water. Spontaneous fission was identified in 1940 by Soviet physicists Georgy Flyorov and Konstantin Petrzhak by their observations of uranium in the Moscow Metro Dinamo station ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
University Of Cambridge
, mottoeng = Literal: From here, light and sacred draughts. Non literal: From this place, we gain enlightenment and precious knowledge. , established = , other_name = The Chancellor, Masters and Scholars of the University of Cambridge , type = Public research university , endowment = £7.121 billion (including colleges) , budget = £2.308 billion (excluding colleges) , chancellor = The Lord Sainsbury of Turville , vice_chancellor = Anthony Freeling , students = 24,450 (2020) , undergrad = 12,850 (2020) , postgrad = 11,600 (2020) , city = Cambridge , country = England , campus_type = , sporting_affiliations = The Sporting Blue , colours = Cambridge Blue , website = , logo = University of Cambridge logo ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Mark Oliphant
Sir Marcus Laurence Elwin Oliphant, (8 October 1901 – 14 July 2000) was an Australian physicist and humanitarian who played an important role in the first experimental demonstration of nuclear fusion and in the development of nuclear weapons. Born and raised in Adelaide, South Australia, Oliphant graduated from the University of Adelaide in 1922. He was awarded an 1851 Exhibition Scholarship in 1927 on the strength of the research he had done on mercury, and went to England, where he studied under Sir Ernest Rutherford at the University of Cambridge's Cavendish Laboratory. There, he used a particle accelerator to fire heavy hydrogen nuclei (deuterons) at various targets. He discovered the respective nuclei of helium-3 (helions) and of tritium (tritons). He also discovered that when they reacted with each other, the particles that were released had far more energy than they started with. Energy had been liberated from inside the nucleus, and he realised that this was a resu ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Nuclear Physics
Nuclear physics is the field of physics that studies atomic nuclei and their constituents and interactions, in addition to the study of other forms of nuclear matter. Nuclear physics should not be confused with atomic physics, which studies the atom as a whole, including its electrons. Discoveries in nuclear physics have led to applications in many fields. This includes nuclear power, nuclear weapons, nuclear medicine and magnetic resonance imaging, industrial and agricultural isotopes, ion implantation in materials engineering, and radiocarbon dating in geology and archaeology. Such applications are studied in the field of nuclear engineering. Particle physics evolved out of nuclear physics and the two fields are typically taught in close association. Nuclear astrophysics, the application of nuclear physics to astrophysics, is crucial in explaining the inner workings of stars and the origin of the chemical elements. History The history of nuclear physics as a discipl ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Gas Giant
A gas giant is a giant planet composed mainly of hydrogen and helium. Gas giants are also called failed stars because they contain the same basic elements as a star. Jupiter and Saturn are the gas giants of the Solar System. The term "gas giant" was originally synonymous with "giant planet". However, in the 1990s, it became known that Uranus and Neptune are really a distinct class of giant planets, being composed mainly of heavier volatile substances (which are referred to as "ices"). For this reason, Uranus and Neptune are now often classified in the separate category of ice giants. Jupiter and Saturn consist mostly of hydrogen and helium, with heavier elements making up between 3 and 13 percent of their mass.The Interior of Jupiter, Guillot et al., in ''Jupiter: The Planet, Satellites and Magnetosphere'', Bagenal et al., editors, Cambridge University Press, 2004 They are thought to consist of an outer layer of compressed molecular hydrogen surrounding a layer of liquid metallic ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
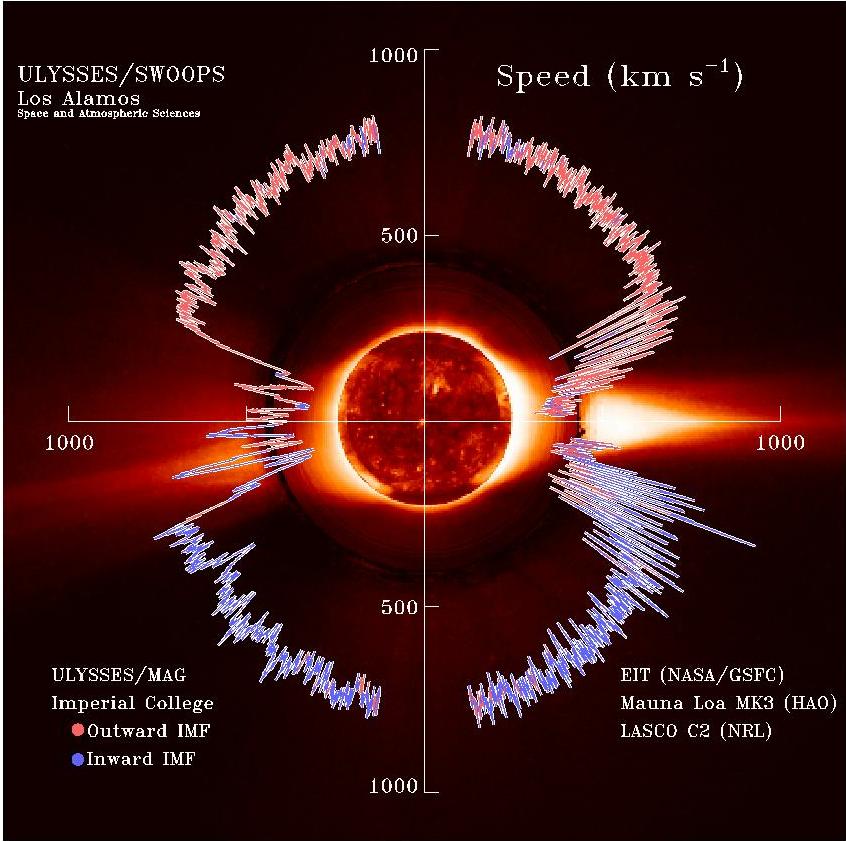
Solar Wind
The solar wind is a stream of charged particles released from the upper atmosphere of the Sun, called the corona. This plasma mostly consists of electrons, protons and alpha particles with kinetic energy between . The composition of the solar wind plasma also includes a mixture of materials found in the solar plasma: trace amounts of heavy ions and atomic nuclei such as C, N, O, Ne, Mg, Si, S, and Fe. There are also rarer traces of some other nuclei and isotopes such as P, Ti, Cr, 54Fe and 56Fe, and 58Ni, 60Ni, and 62Ni. Superposed with the solar-wind plasma is the interplanetary magnetic field. The solar wind varies in density, temperature and speed over time and over solar latitude and longitude. Its particles can escape the Sun's gravity because of their high energy resulting from the high temperature of the corona, which in turn is a result of the coronal magnetic field. The boundary separating the corona from the solar wind is called the Alfvén surface. At a distance of ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Regolith
Regolith () is a blanket of unconsolidated, loose, heterogeneous superficial deposits covering solid rock. It includes dust, broken rocks, and other related materials and is present on Earth, the Moon, Mars, some asteroids, and other terrestrial planets and moons. Etymology The term ''regolith'' combines two Greek words: (), 'blanket', and (), 'rock'. The American geologist George P. Merrill first defined the term in 1897, writing: Earth Earth's regolith includes the following subdivisions and components: * soil or pedolith * alluvium and other transported cover, including that transported by aeolian, glacial, marine, and gravity flow processes. * "saprolith'", generally divided into the ** ''upper saprolite'': completely oxidised bedrock ** ''lower saprolite'': chemically reduced partially weathered rocks ** ''saprock'': fractured bedrock with weathering restricted to fracture margins * volcanic ash and lava flows that are interbedded with unconsolidated material * ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Radioactive
Radioactive decay (also known as nuclear decay, radioactivity, radioactive disintegration, or nuclear disintegration) is the process by which an unstable atomic nucleus loses energy by radiation. A material containing unstable nuclei is considered radioactive. Three of the most common types of decay are alpha decay ( ), beta decay ( ), and gamma decay ( ), all of which involve emitting one or more particles. The weak force is the mechanism that is responsible for beta decay, while the other two are governed by the electromagnetism and nuclear force. A fourth type of common decay is electron capture, in which an unstable nucleus captures an inner electron from one of the electron shells. The loss of that electron from the shell results in a cascade of electrons dropping down to that lower shell resulting in emission of discrete X-rays from the transitions. A common example is iodine-125 commonly used in medical settings. Radioactive decay is a stochastic (i.e. random) process ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Atom
Every atom is composed of a nucleus and one or more electrons bound to the nucleus. The nucleus is made of one or more protons and a number of neutrons. Only the most common variety of hydrogen has no neutrons. Every solid, liquid, gas, and plasma is composed of neutral or ionized atoms. Atoms are extremely small, typically around 100 picometers across. They are so small that accurately predicting their behavior using classical physics, as if they were tennis balls for example, is not possible due to quantum effects. More than 99.94% of an atom's mass is in the nucleus. The protons have a positive electric charge, the electrons have a negative electric charge, and the neutrons have no electric charge. If the number of protons and electrons are equal, then the atom is electrically neutral. If an atom has more or fewer electrons than protons, then it has an overall negative or positive charge, respectively – such atoms are called ions. The electrons of an atom are a ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Nuclear Fusion
Nuclear fusion is a reaction in which two or more atomic nuclei are combined to form one or more different atomic nuclei and subatomic particles ( neutrons or protons). The difference in mass between the reactants and products is manifested as either the release or absorption of energy. This difference in mass arises due to the difference in nuclear binding energy between the atomic nuclei before and after the reaction. Nuclear fusion is the process that powers active or main-sequence stars and other high-magnitude stars, where large amounts of energy are released. A nuclear fusion process that produces atomic nuclei lighter than iron-56 or nickel-62 will generally release energy. These elements have a relatively small mass and a relatively large binding energy per nucleon. Fusion of nuclei lighter than these releases energy (an exothermic process), while the fusion of heavier nuclei results in energy retained by the product nucleons, and the resulting reaction is endo ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Energy Source
Energy development is the field of activities focused on obtaining sources of energy from natural resources. These activities include production of renewable, nuclear, and fossil fuel derived sources of energy, and for the recovery and reuse of energy that would otherwise be wasted. Energy conservation and efficiency measures reduce the demand for energy development, and can have benefits to society with improvements to environmental issues. Societies use energy for transportation, manufacturing, illumination, heating and air conditioning, and communication, for industrial, commercial, and domestic purposes. Energy resources may be classified as primary resources, where the resource can be used in substantially its original form, or as secondary resources, where the energy source must be converted into a more conveniently usable form. Non-renewable resources are significantly depleted by human use, whereas renewable resources are produced by ongoing processes that can susta ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
_0.002_K_region.png)

_Halflife_of_Radionulides_depending_on_Z²_to_A_ratio.png)