|
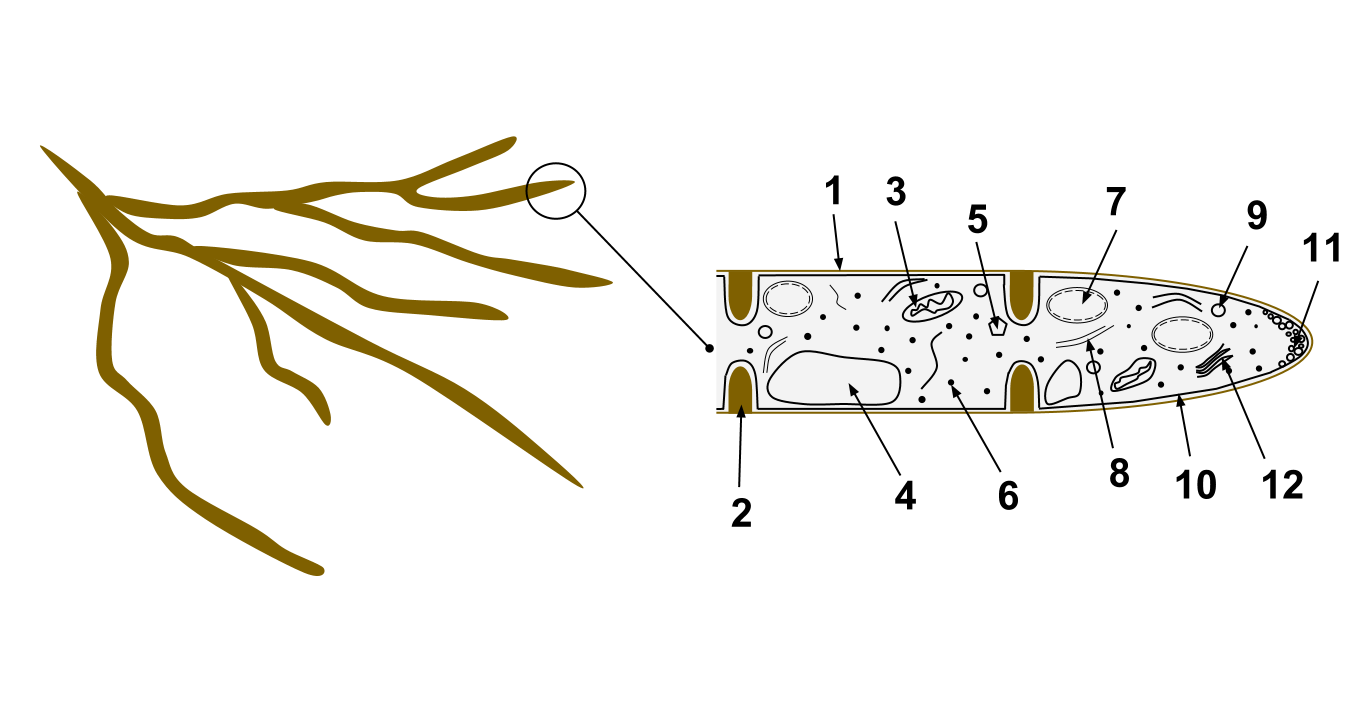
Hanseniaspora Occidentalis
''Hanseniaspora occidentalis'' is a species of yeast in the family Saccharomycetaceae. In its anamorph form, it was called ''Kloeckera javanica''. It has been isolated in the wild from soil samples and vineyards. Samples of a variant have been isolated from orange juice and rotten oranges. It has demonstrated potential as an organism to reduce malic acid in wine production. Taxonomy The yeast was originally isolated by Albert Klöcker in the anamorphic form in 1912 and classified as ''Pseudosaccharomyces occidentalis''. Because the ''Pseudosaccharomyces'' name had already been used since 1906 for an unrelated organism, Alexander Janke proposed an alternative name, ''Klöckeria'', for the genus in 1923, which he corrected in 1928 to ''Kloeckera''. Jacomina Lodder in 1934 found the yeast to be identical to another species, ''Kloeckera jensenii'', also isolated by Klöcker in 1912, and reclassified it as a synonym of ''Kloeckera jensenii''. A similar process occurred with y ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Fungi
A fungus ( : fungi or funguses) is any member of the group of eukaryotic organisms that includes microorganisms such as yeasts and molds, as well as the more familiar mushrooms. These organisms are classified as a kingdom, separately from the other eukaryotic kingdoms, which by one traditional classification include Plantae, Animalia, Protozoa, and Chromista. A characteristic that places fungi in a different kingdom from plants, bacteria, and some protists is chitin in their cell walls. Fungi, like animals, are heterotrophs; they acquire their food by absorbing dissolved molecules, typically by secreting digestive enzymes into their environment. Fungi do not photosynthesize. Growth is their means of mobility, except for spores (a few of which are flagellated), which may travel through the air or water. Fungi are the principal decomposers in ecological systems. These and other differences place fungi in a single group of related organisms, named the ''Eumycota'' (''t ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Budding
Budding or blastogenesis is a type of asexual reproduction in which a new organism develops from an outgrowth or bud due to cell division at one particular site. For example, the small bulb-like projection coming out from the yeast cell is known as a bud. Since the reproduction is asexual, the newly created organism is a clone and excepting mutations is genetically identical to the parent organism. Organisms such as hydra use regenerative cells for reproduction in the process of budding. In hydra, a bud develops as an outgrowth due to repeated cell division at one specific site. These buds develop into tiny individuals and, when fully mature, detach from the parent body and become new independent individuals. Internal budding or endodyogeny is a process of asexual reproduction, favored by parasites such as ''Toxoplasma gondii''. It involves an unusual process in which two daughter cells are produced inside a mother cell, which is then consumed by the offspring prior to their s ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Glycerol
Glycerol (), also called glycerine in British English and glycerin in American English, is a simple triol compound. It is a colorless, odorless, viscous liquid that is sweet-tasting and non-toxic. The glycerol backbone is found in lipids known as glycerides. Because it has antimicrobial and antiviral properties, it is widely used in wound and burn treatments approved by the U.S. Food and Drug Administration. Conversely, it is also used as a bacterial culture medium. It can be used as an effective marker to measure liver disease. It is also widely used as a sweetener in the food industry and as a humectant in pharmaceutical formulations. Because of its three hydroxyl groups, glycerol is miscible with water and is hygroscopic in nature. Structure Although achiral, glycerol is prochiral with respect to reactions of one of the two primary alcohols. Thus, in substituted derivatives, the stereospecific numbering labels the molecule with a "sn-" prefix before the stem name of the m ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Salicin
Salicin is an alcoholic β-glucoside. Salicin is produced in (and named after) willow (''Salix'') bark. It is a biosynthetic precursor to salicylaldehyde. Medicinal aspects Salicin is found in the bark of and leaves of willows, poplars and various other plants. Derivates are found in castoreum. Salicin from meadowsweet was used in the synthesis of aspirin (acetylsalicylic acid), in 1899 by scientists at Bayer. Salicin tastes bitter like quinine Quinine is a medication used to treat malaria and babesiosis. This includes the treatment of malaria due to '' Plasmodium falciparum'' that is resistant to chloroquine when artesunate is not available. While sometimes used for nocturnal le .... Salicin may cause an allergic skin reaction (skin sensitization; category 1). Mild side effects are standard, with rare occurrences of nausea, vomiting, rash, dizziness and breathing problems. Overdose from high quantities of salicin can be toxic, damaging kidneys, causing stomach ul ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Cellobiose
Cellobiose is a disaccharide with the formula (C6H7(OH)4O)2O. It is classified as a reducing sugar. In terms of its chemical structure, it is derived from the condensation of a pair of β-glucose molecules forming a β(1→4) bond. It can be hydrolyzed to glucose enzymatically or with acid. Cellobiose has eight free alcohol (OH) groups, one acetal linkage and one hemiacetal linkage, which give rise to strong inter- and intramolecular hydrogen bonds. It is a white solid. It can be obtained by enzymatic or acidic hydrolysis of cellulose and cellulose-rich materials such as cotton, jute, or paper. Cellobiose can be used as an indicator carbohydrate for Crohn's disease and malabsorption syndrome. Treatment of cellulose with acetic anhydride and sulfuric acid Sulfuric acid (American spelling and the preferred IUPAC name) or sulphuric acid ( Commonwealth spelling), known in antiquity as oil of vitriol, is a mineral acid composed of the elements sulfur, oxygen and hydrogen, ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Assimilation (biology)
Assimilation is the process of absorption of vitamins, minerals, and other chemicals from food as part of the nutrition of an organism. In humans, this is always done with a chemical breakdown (enzymes and acids) and physical breakdown (oral mastication and stomach churning). The second process of bio assimilation is the chemical alteration of substances in the bloodstream by the liver or cellular secretions. Although a few similar compounds can be absorbed in digestion bio assimilation, the bioavailability of many compounds is dictated by this second process since both the liver and cellular secretions can be very specific in their metabolic action (see chirality). This second process is where the absorbed food reaches the cells via the liver. Most foods are composed of largely indigestible components depending on the enzymes and effectiveness of an animal's digestive tract. The most well-known of these indigestible compounds is cellulose; the basic chemical polymer in the m ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Trehalose
Trehalose (from Turkish '' tıgala'' – a sugar derived from insect cocoons + -ose) is a sugar consisting of two molecules of glucose. It is also known as mycose or tremalose. Some bacteria, fungi, plants and invertebrate animals synthesize it as a source of energy, and to survive freezing and lack of water. Extracting trehalose was once a difficult and costly process, but around 2000, the Hayashibara company ( Okayama, Japan) discovered an inexpensive extraction technology from starch. Trehalose has high water retention capabilities, and is used in food, cosmetics and as a drug. A procedure developed in 2017 using trehalose allows sperm storage at room temperatures. Structure Trehalose is a disaccharide formed by a bond between two α-glucose units. It is found in nature as a disaccharide and also as a monomer in some polymers. Two other isomers exist, α,β-trehalose, otherwise known as neotrehalose, and β,β-trehalose (also referred to as isotrehalose). Neotrehalose has ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Raffinose
Raffinose is a trisaccharide composed of galactose, glucose, and fructose. It can be found in beans, cabbage, brussels sprouts, broccoli, asparagus, other vegetables, and whole grains. Raffinose can be hydrolyzed to D-galactose and sucrose by the enzyme α-galactosidase (α-GAL), an enzyme which in the lumen of the human digestive tract is only produced by bacteria in the large intestine. α-GAL also hydrolyzes other α-galactosides such as stachyose, verbascose, and galactinol, if present. The enzyme does not cleave β-linked galactose, as in lactose. Chemical properties The raffinose family of oligosaccharides (RFOs) are alpha-galactosyl derivatives of sucrose, and the most common are the trisaccharide raffinose, the tetrasaccharide stachyose, and the pentasaccharide verbascose. RFOs are almost ubiquitous in the plant kingdom, being found in a large variety of seeds from many different families, and they rank second only to sucrose in abundance as soluble carbohydrate ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Lactose
Lactose is a disaccharide sugar synthesized by galactose and glucose subunits and has the molecular formula C12H22O11. Lactose makes up around 2–8% of milk (by mass). The name comes from ' (gen. '), the Latin word for milk, plus the suffix '' -ose'' used to name sugars. The compound is a white, water-soluble, non-hygroscopic solid with a mildly sweet taste. It is used in the food industry. Structure and reactions Lactose is a disaccharide derived from the condensation of galactose and glucose, which form a β-1→4 glycosidic linkage. Its systematic name is β-D-galactopyranosyl-(1→4)-D-glucose. The glucose can be in either the α-pyranose form or the β-pyranose form, whereas the galactose can only have the β-pyranose form: hence α-lactose and β-lactose refer to the anomeric form of the glucopyranose ring alone. Detection reactions for lactose are the Woehlk- and Fearon's test. Both can be easily used in school experiments to visualise the different lactose content o ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Maltose
} Maltose ( or ), also known as maltobiose or malt sugar, is a disaccharide formed from two units of glucose joined with an α(1→4) bond. In the isomer isomaltose, the two glucose molecules are joined with an α(1→6) bond. Maltose is the two-unit member of the amylose homologous series, the key structural motif of starch. When beta-amylase breaks down starch, it removes two glucose units at a time, producing maltose. An example of this reaction is found in germinating seeds, which is why it was named after malt. Unlike sucrose, it is a reducing sugar. History Maltose was discovered by Augustin-Pierre Dubrunfaut, although this discovery was not widely accepted until it was confirmed in 1872 by Irish chemist and brewer Cornelius O'Sullivan. Its name comes from malt, combined with the suffix ' -ose' which is used in names of sugars. Structure and nomenclature Carbohydrates are generally divided into monosaccharides, oligosaccharides, and polysaccharides depending on the nu ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Galactose
Galactose (, '' galacto-'' + '' -ose'', "milk sugar"), sometimes abbreviated Gal, is a monosaccharide sugar that is about as sweet as glucose, and about 65% as sweet as sucrose. It is an aldohexose and a C-4 epimer of glucose. A galactose molecule linked with a glucose molecule forms a lactose molecule. Galactan is a polymeric form of galactose found in hemicellulose, and forming the core of the galactans, a class of natural polymeric carbohydrates. D-Galactose is also known as brain sugar since it is a component of glycoproteins (oligosaccharide-protein compounds) found in Nerve tissue, nerve tissue. Etymology The word ''galactose'' was coined by Charles Weissman in the mid-19th century and is derived from Greek ''galaktos'' (of milk) and the generic chemical suffix for sugars ''-ose''. The etymology is comparable to that of the word '' lactose'' in that both contain roots meaning "milk sugar". Lactose is a disaccharide of galactose plus glucose. Structure and isomerism Gal ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Sucrose
Sucrose, a disaccharide, is a sugar composed of glucose and fructose subunits. It is produced naturally in plants and is the main constituent of white sugar. It has the molecular formula . For human consumption, sucrose is extracted and refined from either sugarcane or sugar beet. Sugar mills – typically located in tropical regions near where sugarcane is grown – crush the cane and produce raw sugar which is shipped to other factories for refining into pure sucrose. Sugar beet factories are located in temperate climates where the beet is grown, and process the beets directly into refined sugar. The sugar-refining process involves washing the raw sugar crystals before dissolving them into a sugar syrup which is filtered and then passed over carbon to remove any residual colour. The sugar syrup is then concentrated by boiling under a vacuum and crystallized as the final purification process to produce crystals of pure sucrose that are clear, odorless, and sweet. Suga ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |