|
Graphene Production Techniques
A rapidly increasing list of graphene production techniques have been developed to enable graphene's use in commercial applications. Isolated 2D crystals cannot be grown via chemical synthesis beyond small sizes even in principle, because the rapid growth of phonon density with increasing lateral size forces 2D crystallites to bend into the third dimension. However, other routes to 2d materials exist: The early approaches of cleaving multi-layer graphite into single layers or growing it epitaxially by depositing a layer of carbon onto another material have been supplemented by numerous alternatives. In all cases, the graphite must bond to some substrate to retain its 2d shape. Exfoliation As of 2014 exfoliation produced graphene with the lowest number of defects and highest electron mobility. Adhesive tape Andre Geim and Konstantin Novoselov initially used adhesive tape to split graphite into graphene. Achieving single layers typically requires multiple exfoliation steps, ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
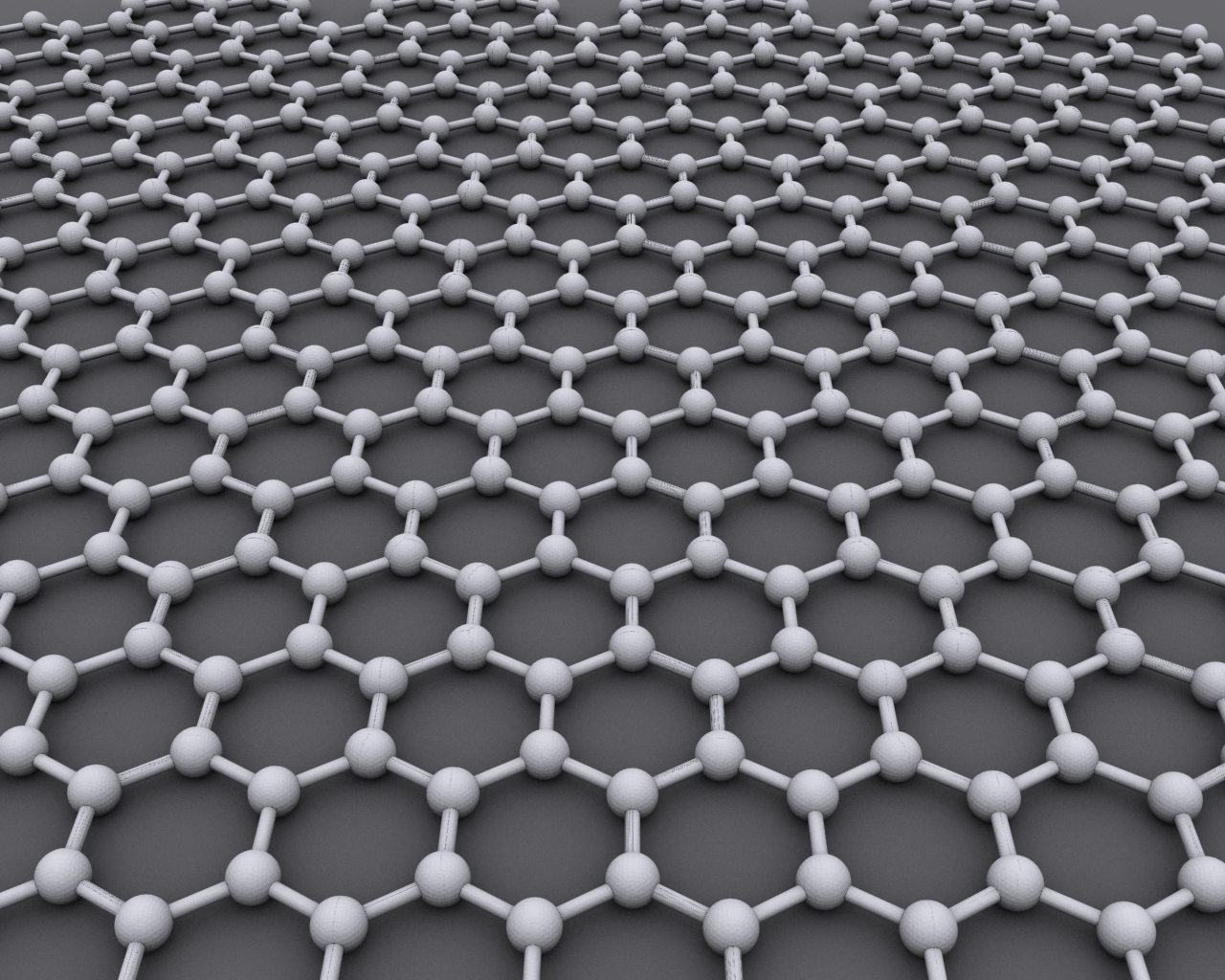
Graphene
Graphene () is an allotrope of carbon consisting of a single layer of atoms arranged in a hexagonal lattice nanostructure. "Carbon nanostructures for electromagnetic shielding applications", Mohammed Arif Poothanari, Sabu Thomas, et al., ''Industrial Applications of Nanomaterials'', 2019. "Carbon nanostructures include various low-dimensional allotropes of carbon including carbon black (CB), carbon fiber, carbon nanotubes (CNTs), fullerene, and graphene." The name is derived from "graphite" and the suffix -ene, reflecting the fact that the allotrope of carbon contains numerous double bonds. Each atom in a graphene sheet is connecte ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Blender
A blender (sometimes called a mixer or liquidiser in British English) is a kitchen appliance, kitchen and laboratory appliance used to mix, crush, purée or emulsion, emulsify food and other substances. A stationary blender consists of a blender container with a rotating metal blade at the bottom, powered by an electric motor that is in the base. Some powerful models can also crush ice and other frozen foods. The newer immersion blender configuration has a motor on top connected by a shaft to a rotating blade at the bottom, which can be used with any container. Characteristics Different blenders have different functions and features but product testing indicates that many blenders, even the less expensive ones, are useful for meeting many consumer needs. Features which consumers consider when purchasing a blender include the following: *large visible measurement marks *ease of use *low noise during usage *power usage (typically 300–1000 watts) *ease of cleaning *option for quic ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Fructose
Fructose, or fruit sugar, is a Ketose, ketonic monosaccharide, simple sugar found in many plants, where it is often bonded to glucose to form the disaccharide sucrose. It is one of the three dietary monosaccharides, along with glucose and galactose, that are absorbed by the gut directly into the blood of the portal vein during digestion. The liver then converts both fructose and galactose into glucose, so that dissolved glucose, known as blood sugar, is the only monosaccharide present in circulating blood. Fructose was discovered by French chemist Augustin-Pierre Dubrunfaut in 1847. The name "fructose" was coined in 1857 by the English chemist William Allen Miller. Pure, dry fructose is a sweet, white, odorless, crystalline solid, and is the most water-soluble of all the sugars. Fructose is found in honey, tree and vine fruits, flowers, Berry, berries, and most List of root vegetables, root vegetables. Commercially, fructose is derived from sugar cane, sugar beets, and maize. Hi ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Glucose
Glucose is a simple sugar with the molecular formula . Glucose is overall the most abundant monosaccharide, a subcategory of carbohydrates. Glucose is mainly made by plants and most algae during photosynthesis from water and carbon dioxide, using energy from sunlight, where it is used to make cellulose in cell walls, the most abundant carbohydrate in the world. In energy metabolism, glucose is the most important source of energy in all organisms. Glucose for metabolism is stored as a polymer, in plants mainly as starch and amylopectin, and in animals as glycogen. Glucose circulates in the blood of animals as blood sugar. The naturally occurring form of glucose is -glucose, while -glucose is produced synthetically in comparatively small amounts and is less biologically active. Glucose is a monosaccharide containing six carbon atoms and an aldehyde group, and is therefore an aldohexose. The glucose molecule can exist in an open-chain (acyclic) as well as ring (cyclic) form. Gluco ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Infrared Laser
A laser is a device that emits light through a process of optical amplification based on the stimulated emission of electromagnetic radiation. The word "laser" is an acronym for "light amplification by stimulated emission of radiation". The first laser was built in 1960 by Theodore H. Maiman at Hughes Research Laboratories, based on theoretical work by Charles Hard Townes and Arthur Leonard Schawlow. A laser differs from other sources of light in that it emits light which is ''coherent''. Spatial coherence allows a laser to be focused to a tight spot, enabling applications such as laser cutting and lithography. Spatial coherence also allows a laser beam to stay narrow over great distances (collimation), enabling applications such as laser pointers and lidar (light detection and ranging). Lasers can also have high temporal coherence, which allows them to emit light with a very narrow spectrum. Alternatively, temporal coherence can be used to produce ultrashort pulses of light ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Heptane
Heptane or ''n''-heptane is the straight-chain alkane with the chemical formula H3C(CH2)5CH3 or C7H16. When used as a test fuel component in anti-knock test engines, a 100% heptane fuel is the zero point of the octane rating scale (the 100 point is 100% iso-octane). Octane number equates to the anti-knock qualities of a comparison mixture of heptane and isooctane which is expressed as the percentage of isooctane in heptane and is listed on pumps for gasoline (petrol) dispensed globally. Uses Heptane (and its many isomers) is widely used in laboratories as a non-polar solvent. As a liquid, it is ideal for transport and storage. In the grease spot test, heptane is used to dissolve an oil spot to show the previous presence of organic compounds on a stained paper. This is done by shaking the stained paper in a heptane solution for about half a minute. Aqueous bromine may be distinguished from aqueous iodine by its appearance after extraction into heptane. In water, both bromine an ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Miscibility
Miscibility () is the property of two substances to mix in all proportions (that is, to fully dissolve in each other at any concentration), forming a homogeneous mixture (a solution). The term is most often applied to liquids but also applies to solids and gases. For example, water and ethanol are miscible because they mix in all proportions. By contrast, substances are said to be immiscible if there are certain proportions in which the mixture does not form a solution. For one example, oil is not soluble in water, so these two solvents are immiscible. As another example, butanone (methyl ethyl ketone) is significantly soluble in water, but these two solvents are also immiscible because in some proportions the mixture will separate into two phases. Organic compounds In organic compounds, the weight percent of hydrocarbon chain often determines the compound's miscibility with water. For example, among the alcohols, ethanol has two carbon atoms and is miscible with water, w ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Surfactant
Surfactants are chemical compounds that decrease the surface tension between two liquids, between a gas and a liquid, or interfacial tension between a liquid and a solid. Surfactants may act as detergents, wetting agents, emulsifiers, foaming agents, or dispersants. The word "surfactant" is a blend of ''surface-active agent'', coined . Agents that increase surface tension are "surface active" in the literal sense but are not called surfactants as their effect is opposite to the common meaning. A common example of surface tension increase is salting out: by adding an inorganic salt to an aqueous solution of a weakly polar substance, the substance will precipitate. The substance may itself be a surfactant – this is one of the reasons why many surfactants are ineffective in sea water. Composition and structure Surfactants are usually organic compounds that are amphiphilic, meaning each molecule contains both a hydrophilic "water-seeking" group (the ''head''), and a hydro ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Ionic Liquid
An ionic liquid (IL) is a salt in the liquid state. In some contexts, the term has been restricted to salts whose melting point is below a specific temperature, such as . While ordinary liquids such as water and gasoline are predominantly made of electrically neutral molecules, ionic liquids are largely made of ions. These substances are variously called liquid electrolytes, ionic melts, ionic fluids, fused salts, liquid salts, or ionic glasses. Ionic liquids have many potential applications. They are powerful solvents and can be used as electrolytes. Salts that are liquid at near-ambient temperature are important for electric battery applications, and have been considered as sealants due to their very low vapor pressure. Any salt that melts without decomposing or vaporizing usually yields an ionic liquid. Sodium chloride (NaCl), for example, melts at into a liquid that consists largely of sodium cations () and chloride anions (). Conversely, when an ionic liquid is cooled, ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
N-methylpyrrolidone
''N''-Methyl-2-pyrrolidone (NMP) is an organic compound consisting of a 5-membered lactam. It is a colorless liquid, although impure samples can appear yellow. It is miscible with water and with most common organic solvents. It also belongs to the class of dipolar aprotic solvents such as dimethylformamide and dimethyl sulfoxide. It is used in the petrochemical and plastics industries as a solvent, exploiting its nonvolatility and ability to dissolve diverse materials. Preparation NMP is produced industrially by a typical ester-to-amide conversion, by treating butyrolactone with methylamine. Alternative routes include the partial hydrogenation of ''N''-methylsuccinimide and the reaction of acrylonitrile with methylamine followed by hydrolysis. About 20,000 to 30,000 tons are produced annually. Applications NMP is used to recover certain hydrocarbons generated in the processing of petrochemicals, such as the recovery of 1,3-butadiene and acetylene. It is used to absorb hydro ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Centrifugation
Centrifugation is a mechanical process which involves the use of the centrifugal force to separate particles from a solution according to their size, shape, density, medium viscosity and rotor speed. The denser components of the mixture migrate away from the axis of the centrifuge, while the less dense components of the mixture migrate towards the axis. Chemists and biologists may increase the effective gravitational force of the test tube so that the precipitate (pellet) will travel quickly and fully to the bottom of the tube. The remaining liquid that lies above the precipitate is called a supernatant or supernate. There is a correlation between the size and density of a particle and the rate that the particle separates from a heterogeneous mixture, when the only force applied is that of gravity. The larger the size and the larger the density of the particles, the faster they separate from the mixture. By applying a larger effective gravitational force to the mixture, like a ce ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Liquid Phase Exfoliation
First demonstrated in 2008, Liquid-phase exfoliation (LPE) is a solution-processing method which is used to convert layered crystals into 2-dimensional nanosheets in large quantities. It is currently one of the pillar methods for producing 2D nanosheets. According to IDTechEx, the family of exfoliation techniques which are directly or indirectly descended from LPE now make up over 60% of global graphene production capacity. This method involves adding powdered layered crystals, for example of graphite, to appropriate solvents and inserting energy, often by ultrasonication, although high-shear mixing is often commonly used. The addition of energy causes a combination of fragmentation and exfoliation resulting in the removal of small nanosheets from the layered crystals. In this way graphite can be converted into large quantities of graphene nanosheets. In general, these nanosheets tend to be a few monolayers thick and of lateral sizes ranging from tens of nanometers to many microns ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |