|
Glycine Receptor Agonist
A glycine receptor agonist is a drug which acts as an agonist of the glycine receptor. Examples Agonists * β-Alanine * D-Alanine * D-Serine * Glycine * Hypotaurine * L-Alanine * L-Proline * L-Serine * Milacemide * Quisqualamine * Sarcosine * Taurine * Hypotaurine PAMs * Ethanol Ethanol (abbr. EtOH; also called ethyl alcohol, grain alcohol, drinking alcohol, or simply alcohol) is an organic compound. It is an Alcohol (chemistry), alcohol with the chemical formula . Its formula can be also written as or (an ethyl ... See also * Glycine receptor antagonist References Drugs acting on the nervous system {{nervous-system-drug-stub ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Drug
A drug is any chemical substance that causes a change in an organism's physiology or psychology when consumed. Drugs are typically distinguished from food and substances that provide nutritional support. Consumption of drugs can be via insufflation (medicine), inhalation, drug injection, injection, smoking, ingestion, absorption (skin), absorption via a dermal patch, patch on the skin, suppository, or sublingual administration, dissolution under the tongue. In pharmacology, a drug is a chemical substance, typically of known structure, which, when administered to a living organism, produces a biological effect. A pharmaceutical drug, also called a medication or medicine, is a chemical substance used to pharmacotherapy, treat, cure, preventive healthcare, prevent, or medical diagnosis, diagnose a disease or to promote well-being. Traditionally drugs were obtained through extraction from medicinal plants, but more recently also by organic synthesis. Pharmaceutical drugs may be used ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Agonist
An agonist is a chemical that activates a receptor to produce a biological response. Receptors are cellular proteins whose activation causes the cell to modify what it is currently doing. In contrast, an antagonist blocks the action of the agonist, while an inverse agonist causes an action opposite to that of the agonist. Etymology From the Greek αγωνιστής (agōnistēs), contestant; champion; rival < αγων (agōn), contest, combat; exertion, struggle < αγω (agō), I lead, lead towards, conduct; drive Types of agonists can be activated by either endogenous agonists (such as |
Glycine Receptor
The glycine receptor (abbreviated as GlyR or GLR) is the receptor of the amino acid neurotransmitter glycine. GlyR is an ionotropic receptor that produces its effects through chloride current. It is one of the most widely distributed inhibitory receptors in the central nervous system and has important roles in a variety of physiological processes, especially in mediating inhibitory neurotransmission in the spinal cord and brainstem. The receptor can be activated by a range of simple amino acids including glycine, β-alanine and taurine, and can be selectively blocked by the high-affinity competitive antagonist strychnine. Caffeine is a competitive antagonist of GlyR. Gephyrin has been shown to be necessary for GlyR clustering at inhibitory synapses. GlyR is known to colocalize with the GABAA receptor on some hippocampal neurons. Nevertheless, some exceptions can occur in the central nervous system where the GlyR α1 subunit and gephyrin, its anchoring protein, are not foun ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
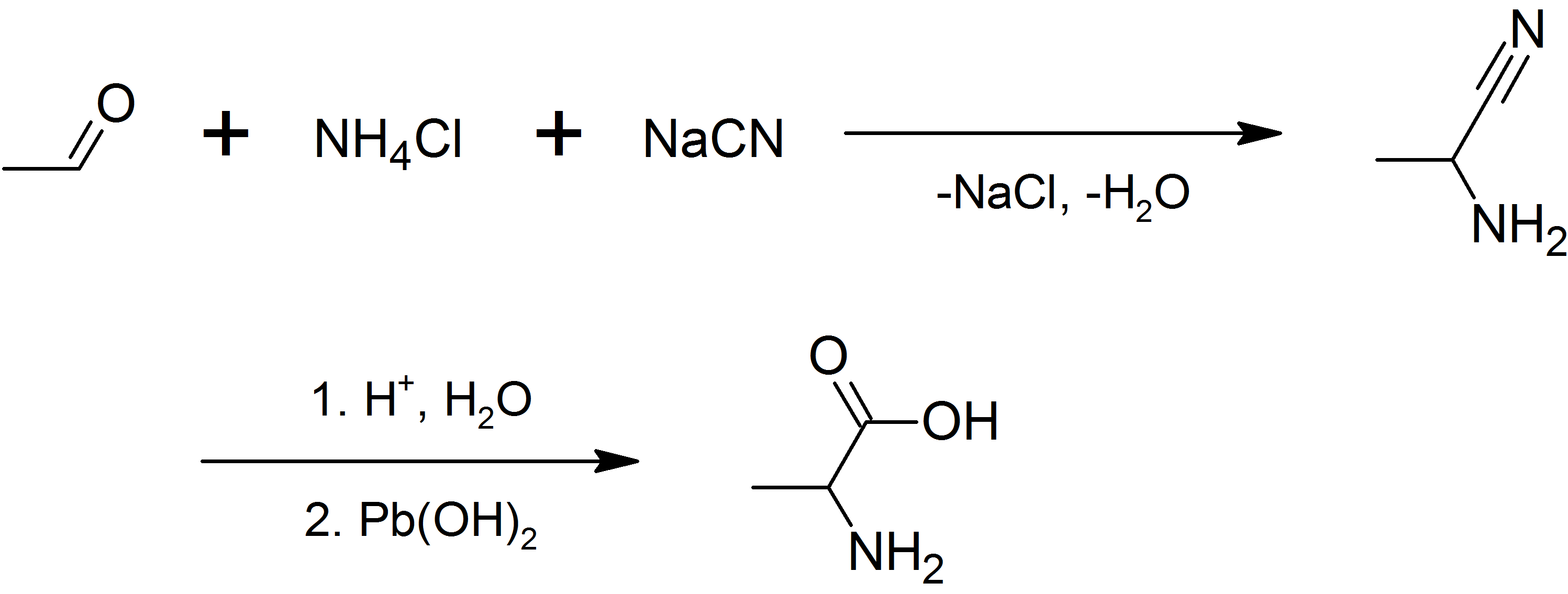
Alanine
Alanine (symbol Ala or A), or α-alanine, is an α-amino acid that is used in the biosynthesis of proteins. It contains an amine group and a carboxylic acid group, both attached to the central carbon atom which also carries a methyl group side chain. Consequently, its IUPAC systematic name is 2-aminopropanoic acid, and it is classified as a nonpolar, aliphatic α-amino acid. Under biological conditions, it exists in its zwitterionic form with its amine group protonated (as −NH3+) and its carboxyl group deprotonated (as −CO2−). It is non-essential to humans as it can be synthesised metabolically and does not need to be present in the diet. It is encoded by all codons starting with GC (GCU, GCC, GCA, and GCG). The L-isomer of alanine (left-handed) is the one that is incorporated into proteins. L-alanine is second only to leucine in rate of occurrence, accounting for 7.8% of the primary structure in a sample of 1,150 proteins. The right-handed form, D-alanine, occurs in p ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Serine
Serine (symbol Ser or S) is an α-amino acid that is used in the biosynthesis of proteins. It contains an α-amino group (which is in the protonated − form under biological conditions), a carboxyl group (which is in the deprotonated − form under biological conditions), and a side chain consisting of a hydroxymethyl group, classifying it as a polar amino acid. It can be synthesized in the human body under normal physiological circumstances, making it a nonessential amino acid. It is encoded by the codons UCU, UCC, UCA, UCG, AGU and AGC. Occurrence This compound is one of the naturally occurring proteinogenic amino acids. Only the L-stereoisomer appears naturally in proteins. It is not essential to the human diet, since it is synthesized in the body from other metabolites, including glycine. Serine was first obtained from silk protein, a particularly rich source, in 1865 by Emil Cramer. Its name is derived from the Latin for silk, ''sericum''. Serine's structure was estab ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Glycine
Glycine (symbol Gly or G; ) is an amino acid that has a single hydrogen atom as its side chain. It is the simplest stable amino acid (carbamic acid is unstable), with the chemical formula NH2‐ CH2‐ COOH. Glycine is one of the proteinogenic amino acids. It is encoded by all the codons starting with GG (GGU, GGC, GGA, GGG). Glycine is integral to the formation of alpha-helices in secondary protein structure due to its compact form. For the same reason, it is the most abundant amino acid in collagen triple-helices. Glycine is also an inhibitory neurotransmitter – interference with its release within the spinal cord (such as during a ''Clostridium tetani'' infection) can cause spastic paralysis due to uninhibited muscle contraction. It is the only achiral proteinogenic amino acid. It can fit into hydrophilic or hydrophobic environments, due to its minimal side chain of only one hydrogen atom. History and etymology Glycine was discovered in 1820 by the French chemist He ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Hypotaurine
Hypotaurine is a sulfinic acid that is an intermediate in the biosynthesis of taurine. Like taurine, it also acts as an endogenous neurotransmitter via action on the glycine receptors. It is an osmolyte with antioxidant properties. Hypotaurine is derived from cysteine (and homocysteine). In mammals, the biosynthesis of hypotaurine from cysteine occurs in the pancreas. In the cysteine sulfinic acid pathway, cysteine is first oxidized to its sulfinic acid, catalyzed by the enzyme cysteine dioxygenase Cysteine dioxygenase (CDO) is a non-heme iron enzyme that catalyzes the conversion of L-cysteine to cysteine sulfinic acid (cysteine sulfinate). CDO plays an important role in cysteine catabolism, regulating intracellular levels of cysteine and res .... Cysteine sulfinic acid, in turn, is decarboxylated by sulfinoalanine decarboxylase to form hypotaurine. Hypotaurine is enzymatically oxidized to yield taurine by hypotaurine dehydrogenase. References Amines Sulfinic acids ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Proline
Proline (symbol Pro or P) is an organic acid classed as a proteinogenic amino acid (used in the biosynthesis of proteins), although it does not contain the amino group but is rather a secondary amine. The secondary amine nitrogen is in the protonated form (NH2+) under biological conditions, while the carboxyl group is in the deprotonated −COO− form. The "side chain" from the α carbon connects to the nitrogen forming a pyrrolidine loop, classifying it as a aliphatic amino acid. It is non-essential in humans, meaning the body can synthesize it from the non-essential amino acid L-glutamate. It is encoded by all the codons starting with CC (CCU, CCC, CCA, and CCG). Proline is the only proteinogenic secondary amino acid which is a secondary amine, as the nitrogen atom is attached both to the α-carbon and to a chain of three carbons that together form a five-membered ring. History and etymology Proline was first isolated in 1900 by Richard Willstätter who obtained the amino ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Milacemide
Milacemide (INN) is an MAO-B inhibitor and glycine prodrug. It has been studied for its effects on human memory and as a potential treatment for the symptoms of Alzheimer's disease. Early clinical trials did not show positive results however, and the drug is now abandoned and it is sold as a nonprescription drug or supplement. While milacemide is not an amino-acid, it acts similarly to glycine Glycine (symbol Gly or G; ) is an amino acid that has a single hydrogen atom as its side chain. It is the simplest stable amino acid ( carbamic acid is unstable), with the chemical formula NH2‐ CH2‐ COOH. Glycine is one of the proteinog ... in the brain. References Abandoned drugs Amino acid derivatives Monoamine oxidase inhibitors Glycine receptor agonists NMDA receptor agonists Prodrugs {{nervous-system-drug-stub ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Quisqualamine
Quisqualamine is the α-decarboxylated analogue of quisqualic acid, as well as a relative of the neurotransmitters glutamate and γ-aminobutyric acid (GABA). α-Decarboxylation of excitatory amino acids can produce derivatives with inhibitory effects. Indeed, unlike quisqualic acid, quisqualamine has central depressant and neuroprotective properties and appears to act predominantly as an agonist of the GABAA receptor and also to a lesser extent as an agonist of the glycine receptor, due to the facts that its actions are inhibited ''in vitro'' by GABAA antagonists like bicuculline and picrotoxin and by the glycine antagonist strychnine, respectively. Mg2+ and DL-AP5, NMDA receptor blockers, CNQX, an antagonist of both the AMPA and kainate receptors, and 2-hydroxysaclofen, a GABAB receptor antagonist, do not affect quisqualamine's actions ''in vitro'', suggesting that it does not directly affect the ionotropic glutamate receptors or the GABAB receptor in any way. Whether it ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Sarcosine
Sarcosine, also known as ''N''-methylglycine, or monomethylglycine, is a monopeptide with the formula CH3N(H)CH2CO2H. It exists at neutral pH as the zwitterion CH3N+(H)2CH2CO2−, which can be obtained as a white, water-soluble powder. Like some amino acids, sarcosine converts to a cation at low pH and an anion at high pH, with the respective formulas CH3N+(H)2CH2CO2H and CH3N(H)CH2CO2−. Sarcosine is a close relative of glycine, with a secondary amine in place of the primary amine. Sarcosine is ubiquitous in biological materials. It is used in manufacturing biodegradable surfactants and toothpastes as well as in other applications. Sarcosine is sweet to the taste. Biochemistry Sarcosine is an intermediate and byproduct in glycine synthesis and degradation. Sarcosine is metabolized to glycine by the enzyme sarcosine dehydrogenase, while glycine-''N''-methyl transferase generates sarcosine from glycine. Sarcosine is an amino acid derivative that is naturally found in muscles ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |