|
Glassmaking Companies Of Japan
Glass production involves two main methods – the float glass process that produces sheet glass, and glassblowing that produces bottles and other containers. It has been done in a variety of ways during the history of glass. Glass container production Broadly, modern glass container factories are three-part operations: the "batch house", the "hot end", and the "cold end". The batch house handles the raw materials; the hot end handles the manufacture proper—the forehearth, forming machines, and annealing ovens; and the cold end handles the product-inspection and packaging equipment. Batch processing system (batch house) Batch processing is one of the initial steps of the glass-making process. The batch house simply houses the raw materials in large silos (fed by truck or railcar), and holds anywhere from 1–5 days of material. Some batch systems include material processing such as raw material screening/sieve, drying, or pre-heating (i.e. cullet). Whether automated or ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Glass Bottles
A glass bottle is a bottle made from glass. Glass bottles can vary in size considerably, but are most commonly found in sizes ranging between about 200 millilitres and 1.5 litres. Common uses for glass bottles include food condiments, soda, liquor, cosmetics, pickling and preservatives; they are occasionally also notably used for the informal distribution of notes. These types of bottles are utilitarian and serve a purpose in commercial industries. History Glass bottles and glass jars are found in many households worldwide. The first glass bottles were produced in Mesopotamia around 1500 B.C., and in the Roman Empire around 1 AD. America's glass bottle and glass jar industry was born in the early 1600s, when settlers in Jamestown built the first glass-melting furnace. The invention of the automatic glass bottle-blowing machine in 1903 industrialized the process of making bottles. Manufacture The earliest bottles or vessels were made by ancient man. Ingredients were melte ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Pneumatics
Pneumatics (from Greek ‘wind, breath’) is a branch of engineering that makes use of gas or pressurized air. Pneumatic systems used in industry are commonly powered by compressed air or compressed inert gases. A centrally located and electrically-powered compressor powers cylinders, air motors, pneumatic actuators, and other pneumatic devices. A pneumatic system controlled through manual or automatic solenoid valves is selected when it provides a lower cost, more flexible, or safer alternative to electric motors, and hydraulic actuators. Pneumatics also has applications in dentistry, construction, mining, and other areas. Gases used in pneumatic systems Pneumatic systems in fixed installations, such as factories, use compressed air because a sustainable supply can be made by compressing atmospheric air. The air usually has moisture removed, and a small quantity of oil is added at the compressor to prevent corrosion and lubricate mechanical components. Factory-plumb ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Vitreous Enamel
Vitreous enamel, also called porcelain enamel, is a material made by fusing powdered glass to a substrate by firing, usually between . The powder melts, flows, and then hardens to a smooth, durable vitreous coating. The word comes from the Latin , meaning "glass". Enamel can be used on metal, glass, ceramics, stone, or any material that will withstand the fusing temperature. In technical terms fired enamelware is an integrated layered composite of glass and another material (or more glass). The term "enamel" is most often restricted to work on metal, which is the subject of this article. Essentially the same technique used with other bases is known by different terms: on glass as ''enamelled glass'', or "painted glass", and on pottery it is called ''overglaze decoration'', "overglaze enamels" or "enamelling". The craft is called "enamelling", the artists "enamellers" and the objects produced can be called "enamels". Enamelling is an old and widely adopted technology, for mo ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Screen-printing
Screen printing is a printing technique where a mesh is used to transfer ink (or dye) onto a substrate, except in areas made impermeable to the ink by a blocking stencil. A blade or squeegee is moved across the screen to fill the open mesh apertures with ink, and a reverse stroke then causes the screen to touch the substrate momentarily along a line of contact. This causes the ink to wet the substrate and be pulled out of the mesh apertures as the screen springs back after the blade has passed. One colour is printed at a time, so several screens can be used to produce a multi-coloured image or design. Traditionally, silk was used in the process. Currently, synthetic threads are commonly used in the screen printing process. The most popular mesh in general use is made of polyester. There are special-use mesh materials of nylon and stainless steel available to the screen-printer. There are also different types of mesh size which will determine the outcome and look of the fin ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Refractory
In materials science, a refractory material or refractory is a material that is resistant to decomposition by heat, pressure, or chemical attack, and retains strength and form at high temperatures. Refractories are polycrystalline, polyphase, inorganic, non-metallic, porous, and heterogeneous. They are typically composed of oxides or carbides, nitrides etc. of the following materials: silicon, aluminium, magnesium, calcium, boron, chromium and zirconium. ASTM C71 defines refractories as "...non-metallic materials having those chemical and physical properties that make them applicable for structures, or as components of systems, that are exposed to environments above ." Refractory materials are used in furnaces, kilns, incinerators, and reactors. Refractories are also used to make crucibles and moulds for casting glass and metals and for surfacing flame deflector systems for rocket launch structures. Today, the iron- and steel-industry and metal casting sectors use appr ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Conveyor
A conveyor system is a common piece of mechanical handling equipment that moves materials from one location to another. Conveyors are especially useful in applications involving the transport of heavy or bulky materials. Conveyor systems allow quick and efficient transport for a wide variety of materials, which make them very popular in the material handling and packaging industries. They also have popular consumer applications, as they are often found in supermarkets and airports, constituting the final leg of item/ bag delivery to customers. Many kinds of conveying systems are available and are used according to the various needs of different industries. There are chain conveyors (floor and overhead) as well. Chain conveyors consist of enclosed tracks, I-Beam, towline, power & free, and hand pushed trolleys. Industries where used Conveyor systems are used widespread across a range of industries due to the numerous benefits they provide. * Conveyors are able to safely tra ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Emulsion
An emulsion is a mixture of two or more liquids that are normally immiscible (unmixable or unblendable) owing to liquid-liquid phase separation. Emulsions are part of a more general class of two-phase systems of matter called colloids. Although the terms ''colloid'' and ''emulsion'' are sometimes used interchangeably, ''emulsion'' should be used when both phases, dispersed and continuous, are liquids. In an emulsion, one liquid (the dispersed phase) is dispersed in the other (the continuous phase). Examples of emulsions include vinaigrettes, homogenized milk, liquid biomolecular condensates, and some cutting fluids for metal working. Two liquids can form different types of emulsions. As an example, oil and water can form, first, an oil-in-water emulsion, in which the oil is the dispersed phase, and water is the continuous phase. Second, they can form a water-in-oil emulsion, in which water is the dispersed phase and oil is the continuous phase. Multiple emulsions are also pos ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
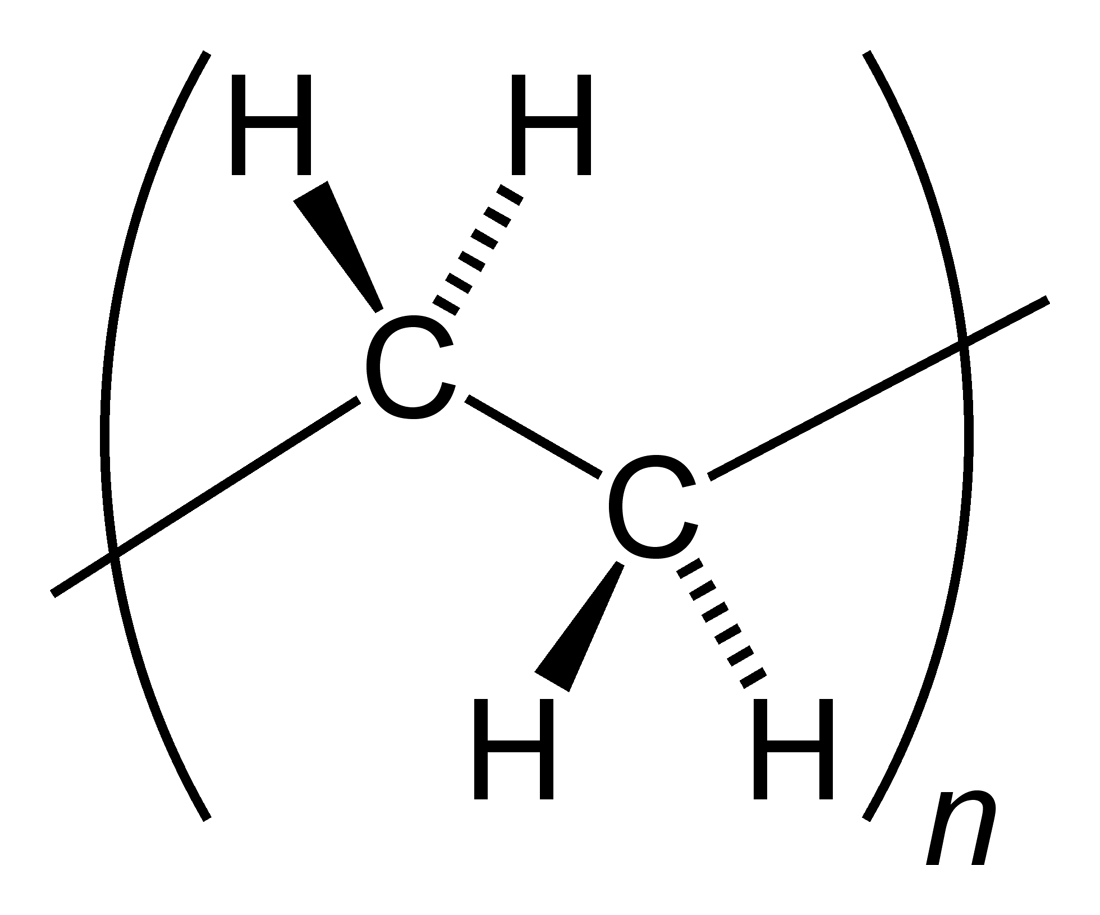
Polyethylene
Polyethylene or polythene (abbreviated PE; IUPAC name polyethene or poly(methylene)) is the most commonly produced plastic. It is a polymer, primarily used for packaging ( plastic bags, plastic films, geomembranes and containers including bottles, etc.). , over 100 million tonnes of polyethylene resins are being produced annually, accounting for 34% of the total plastics market. Many kinds of polyethylene are known, with most having the chemical formula (C2H4)''n''. PE is usually a mixture of similar polymers of ethylene, with various values of ''n''. It can be ''low-density'' or ''high-density'': low-density polyethylene is extruded using high pressure () and high temperature (), while high-density polyethylene is extruded using low pressure () and low temperature (). Polyethylene is usually thermoplastic, but it can be modified to become thermosetting instead, for example, in cross-linked polyethylene. History Polyethylene was first synthesized by the German chemist Hans ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Titanium Tetrachloride
Titanium tetrachloride is the inorganic compound with the formula . It is an important intermediate in the production of titanium metal and the pigment titanium dioxide. is a volatile liquid. Upon contact with humid air, it forms thick clouds of titanium dioxide () and hydrochloric acid, a reaction that was formerly exploited for use in smoke machines. It is sometimes referred to as "tickle" or "tickle 4" due to the phonetic resemblance of its molecular formula () to the word. Properties and structure is a dense, colourless distillable liquid, although crude samples may be yellow or even red-brown. It is one of the rare transition metal halides that is a liquid at room temperature, being another example. This property reflects the fact that molecules of weakly self-associate. Most metal chlorides are polymers, wherein the chloride atoms bridge between the metals. Its melting and boiling points are similar to those of . has a "closed" electronic shell, with the same numbe ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Stannic Chloride
Tin(IV) chloride, also known as tin tetrachloride or stannic chloride, is an inorganic compound with the formula Sn Cl4. It is a colorless hygroscopic liquid, which fumes on contact with air. It is used as a precursor to other tin compounds. It was first discovered by Andreas Libavius (1550–1616) and was known as ''spiritus fumans libavii''. Preparation It is prepared from reaction of chlorine gas with tin at . : Sn + 2 Cl2 → SnCl4 Structure Anhydrous tin(IV) chloride solidifies at −33 °C to give monoclinic crystals with the P21/c space group. It is isostructural with SnBr4. The molecules adopt near-perfect tetrahedral symmetry with average Sn–Cl distances of 227.9(3) pm. Reactions Tin(IV) chloride is well known as a Lewis acid. Thus it forms hydrates. The pentahydrate SnCl4·5H2O was formerly known as butter of tin. They all consist of nCl4(H2O)2molecules together with varying amounts of water of crystallization. The additional water molecules link togeth ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Tin(IV) Oxide
Tin(IV) oxide, also known as stannic oxide, is the inorganic compound with the formula SnO2. The mineral form of SnO2 is called cassiterite, and this is the main ore of tin. With many other names, this oxide of tin is an important material in tin chemistry. It is a colourless, diamagnetic, amphoteric solid. Structure Tin(IV) oxide crystallises with the rutile structure. As such the tin atoms are six coordinate and the oxygen atoms three coordinate. SnO2 is usually regarded as an oxygen-deficient n-type semiconductor. Hydrous forms of SnO2 have been described as stannic acid. Such materials appear to be hydrated particles of SnO2 where the composition reflects the particle size. Preparation Tin(IV) oxide occurs naturally. Synthetic tin(IV) oxide is produced by burning tin metal in air. Annual production is in the range of 10 kilotons. SnO2 is reduced industrially to the metal with carbon in a reverberatory furnace at 1200–1300 °C. Amphoterism Although SnO2 is insolubl ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Lehr (glassmaking)
In the manufacture of float glass, a lehr oven is a long kiln with an end-to-end temperature gradient, which is used for annealing newly made glass objects that are transported through the temperature gradient either on rollers or on a conveyor belt. The annealing renders glass into a stronger material with fewer internal stresses, and with a lower probability of breaking. The rapid cooling of molten glass results in an uneven temperature distribution throughout the material. This temperature differential results in mechanical stresses throughout the molten glass, which may be sufficient to cause the material to crack as it cools to ambient temperature or to make it susceptible to cracking during later use, either spontaneously or due to mechanical or thermal shock. To prevent such material weaknesses, objects made from molten glass are annealed by gradual cooling in a lehr oven, from the annealing point, a temperature just below the solidification temperature of the glass. In t ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |