|
Feklichevite
Feklichevite is a rare mineral of the eudialyte group with the formula Na11Ca9(Fe3+,Fe2+)2Zr3NbSi(Si3O9)2(Si9O27)2.Pekov, I.V., Ekimenkova, I.A., Chukanov, N.V., Rastsvetaeva, R.K., Kononkova, N.N., Pekova, N.A., and Zadov, A.E., 2001. Feklichevite Na11Ca9(Fe3+,Fe2+)2Zr3Nb i25O73OH,H2O,Cl,O)5, a new mineral of the eudialyte group from Kovdor Massif, Kola peninsula. Zapiski Vserossijskogo Mineralogicheskogo Obshchestva 130(3), 55-65Mindat, Feklichevite, http://www.mindat.org/min-11029.html The original formula was extended to show the presence of cyclic silicate groups and presence of silicon at the M4 site, according to the nomenclature of eudialyte group.Johnsen, O., Ferraris, G., Gault, R.A., Grice, D.G., Kampf, A.R., and Pekov, I.V., 2003. The nomenclature of eudialyte-group minerals. The Canadian Mineralogist 41, 785-794 When compared to other minerals of the group, feklichevite characterizes in the presence of ferric iron (thus similar to ikranite, mogovidite and fengchengite) ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Eudialyte Group
Eudialyte group is a group of complex trigonal zircono- and, more rarely, titanosilicate minerals with general formula (1)N(2)N(3)N(4)N(5)sub>3 (1a)M(1b)sub>3M(2)3M(4)Z3 i24O72'4X2, where N(1) and N(2) and N(3) and N(5) = Na+ and more rarely H3O+ or H2O, N(4) = Na+, Sr2+, Mn2+ and more rarely H3O+ or H2O or K+ or Ca2+ or REE3+ (rare earth elements), M(1) and M(1b) = Ca2+, M(1a) = Ca2+ or Mn2+ or Fe2+, M(2) = Fe (both II and III), Mn and rarely Na+, K+ or Zr4+, M(3) = Si, Nb and rarely W, Ti and [] (vacancy defect, vacancy), M(4) = Si and or rarely [], Z Zr4+ and or rarely Ti4+, and X = OH−, Cl− and more rarely CO32− or F−. Some of the eudialyte-like structures can even be more complex, however, in general, its typical feature is the presence of i3O9sup>6− and i9O27sup>18− ring silicate groups. Space group is usually ''R''3''m'' or ''R''-3''m'' but may be reduced to ''R''3 due to cation ordering. Like other zirconosilicates, the eudialyte group minerals pos ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Cyclosilicate
Silicate minerals are rock-forming minerals made up of silicate groups. They are the largest and most important class of minerals and make up approximately 90 percent of Earth's crust. In mineralogy, silica (silicon dioxide, ) is usually considered a silicate mineral. Silica is found in nature as the mineral quartz, and its polymorphism (materials science), polymorphs. On Earth, a wide variety of silicate minerals occur in an even wider range of combinations as a result of the processes that have been forming and re-working the crust for billions of years. These processes include partial melting, crystallization, fractionation, metamorphism, weathering, and diagenesis. Living organisms also contribute to this carbonate–silicate cycle, geologic cycle. For example, a type of plankton known as diatoms construct their exoskeletons ("frustules") from silica extracted from seawater. The frustules of dead diatoms are a major constituent of deep ocean sediment, and of diatomaceous e ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Aegirine
Aegirine is a member of the clino pyroxene group of inosilicate minerals. Aegirine is the sodium endmember of the aegirine- augite series. Aegirine has the chemical formula Na Fe Si2 O6 in which the iron is present as Fe3+. In the aegirine-augite series the sodium is variably replaced by calcium with iron(II) and magnesium replacing the iron(III) to balance the charge. Aluminium also substitutes for the iron(III). Acmite is a fibrous, green-colored variety. Aegirine occurs as dark green monoclinic prismatic crystals. It has a glassy luster and perfect cleavage. Its Mohs hardness varies from 5 to 6, and its specific gravity is between 3.2 and 3.4. This mineral commonly occurs in alkalic igneous rocks, nepheline syenites, carbonatites and pegmatites. It also appears in regionally metamorphosed schists, gneisses, and iron formations; in blueschist facies rocks, and from sodium metasomatism in granulites. It may occur as an authigenic mineral in shales and marls. It occurs in ass ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
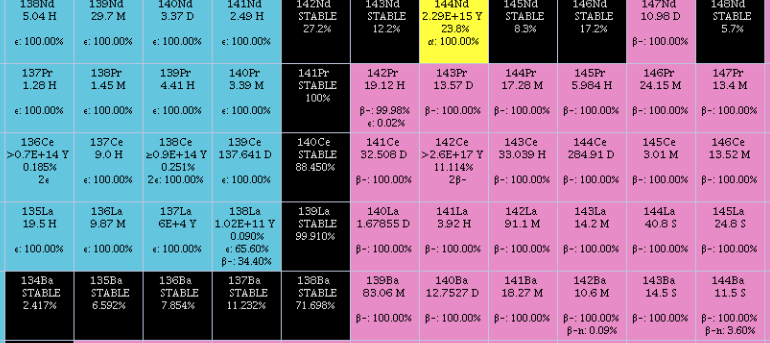
Lanthanum
Lanthanum is a chemical element with the symbol La and atomic number 57. It is a soft, ductile, silvery-white metal that tarnishes slowly when exposed to air. It is the eponym of the lanthanide series, a group of 15 similar elements between lanthanum and lutetium in the periodic table, of which lanthanum is the first and the prototype. Lanthanum is traditionally counted among the rare earth elements. Like most other rare earth elements, the usual oxidation state is +3. Lanthanum has no biological role in humans but is essential to some bacteria. It is not particularly toxic to humans but does show some antimicrobial activity. Lanthanum usually occurs together with cerium and the other rare earth elements. Lanthanum was first found by the Swedish chemist Carl Gustaf Mosander in 1839 as an impurity in cerium nitrate – hence the name ''lanthanum'', from the Ancient Greek (), meaning 'to lie hidden'. Although it is classified as a rare earth element, lanthanum is the 28th most abund ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Cerium
Cerium is a chemical element with the symbol Ce and atomic number 58. Cerium is a soft, ductile, and silvery-white metal that tarnishes when exposed to air. Cerium is the second element in the lanthanide series, and while it often shows the +3 oxidation state characteristic of the series, it also has a stable +4 state that does not oxidize water. It is also considered one of the rare-earth elements. Cerium has no known biological role in humans but is not particularly toxic, except with intense or continued exposure. Despite always occurring in combination with the other rare-earth elements in minerals such as those of the monazite and bastnäsite groups, cerium is easy to extract from its ores, as it can be distinguished among the lanthanides by its unique ability to be oxidized to the +4 state in aqueous solution. It is the most common of the lanthanides, followed by neodymium, lanthanum, and praseodymium. It is the 25th-most abundant element, making up 66 ppm of the Ear ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
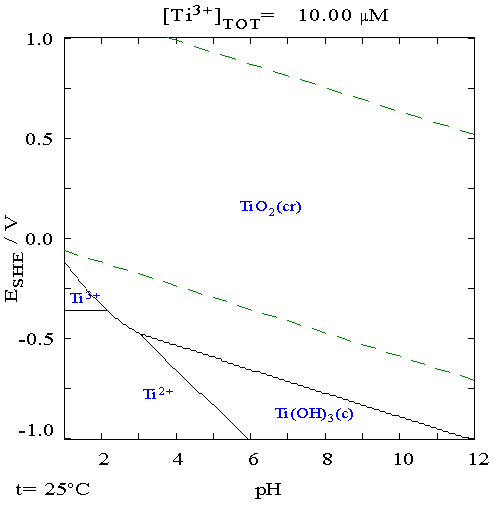
Titanium
Titanium is a chemical element with the symbol Ti and atomic number 22. Found in nature only as an oxide, it can be reduced to produce a lustrous transition metal with a silver color, low density, and high strength, resistant to corrosion in sea water, aqua regia, and chlorine. Titanium was discovered in Cornwall, Great Britain, by William Gregor in 1791 and was named by Martin Heinrich Klaproth after the Titans of Greek mythology. The element occurs within a number of minerals, principally rutile and ilmenite, which are widely distributed in the Earth's crust and lithosphere; it is found in almost all living things, as well as bodies of water, rocks, and soils. The metal is extracted from its principal mineral ores by the Kroll and Hunter processes. The most common compound, titanium dioxide, is a popular photocatalyst and is used in the manufacture of white pigments. Other compounds include titanium tetrachloride (TiCl4), a component of smoke screens and catalysts; and ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Strontium
Strontium is the chemical element with the symbol Sr and atomic number 38. An alkaline earth metal, strontium is a soft silver-white yellowish metallic element that is highly chemically reactive. The metal forms a dark oxide layer when it is exposed to air. Strontium has physical and chemical properties similar to those of its two vertical neighbors in the periodic table, calcium and barium. It occurs naturally mainly in the minerals celestine and strontianite, and is mostly mined from these. Both strontium and strontianite are named after Strontian, a village in Scotland near which the mineral was discovered in 1790 by Adair Crawford and William Cruickshank; it was identified as a new element the next year from its crimson-red flame test color. Strontium was first isolated as a metal in 1808 by Humphry Davy using the then newly discovered process of electrolysis. During the 19th century, strontium was mostly used in the production of sugar from sugar beets (see strontian p ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Hafnium
Hafnium is a chemical element with the symbol Hf and atomic number 72. A lustrous, silvery gray, tetravalent transition metal, hafnium chemically resembles zirconium and is found in many zirconium minerals. Its existence was predicted by Dmitri Mendeleev in 1869, though it was not identified until 1923, by Dirk Coster and George de Hevesy, making it the penultimate stable element to be discovered (the last being rhenium in 1925). Hafnium is named after , the Latin name for Copenhagen, where it was discovered. Hafnium is used in filaments and electrodes. Some semiconductor fabrication processes use its oxide for integrated circuits at 45 nanometers and smaller feature lengths. Some superalloys used for special applications contain hafnium in combination with niobium, titanium, or tungsten. Hafnium's large neutron capture cross section makes it a good material for neutron absorption in control rods in nuclear power plants, but at the same time requires that it be removed from th ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Fluorine
Fluorine is a chemical element with the symbol F and atomic number 9. It is the lightest halogen and exists at standard conditions as a highly toxic, pale yellow diatomic gas. As the most electronegative reactive element, it is extremely reactive, as it reacts with all other elements except for the light inert gases. Among the elements, fluorine ranks 24th in universal abundance and 13th in terrestrial abundance. Fluorite, the primary mineral source of fluorine which gave the element its name, was first described in 1529; as it was added to metal ores to lower their melting points for smelting, the Latin verb meaning 'flow' gave the mineral its name. Proposed as an element in 1810, fluorine proved difficult and dangerous to separate from its compounds, and several early experimenters died or sustained injuries from their attempts. Only in 1886 did French chemist Henri Moissan isolate elemental fluorine using low-temperature electrolysis, a process still employed for modern pr ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Manganese
Manganese is a chemical element with the symbol Mn and atomic number 25. It is a hard, brittle, silvery metal, often found in minerals in combination with iron. Manganese is a transition metal with a multifaceted array of industrial alloy uses, particularly in stainless steels. It improves strength, workability, and resistance to wear. Manganese oxide is used as an oxidising agent; as a rubber additive; and in glass making, fertilisers, and ceramics. Manganese sulfate can be used as a fungicide. Manganese is also an essential human dietary element, important in macronutrient metabolism, bone formation, and free radical defense systems. It is a critical component in dozens of proteins and enzymes. It is found mostly in the bones, but also the liver, kidneys, and brain. In the human brain, the manganese is bound to manganese metalloproteins, most notably glutamine synthetase in astrocytes. Manganese was first isolated in 1774. It is familiar in the laboratory in the form of the ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Hematite
Hematite (), also spelled as haematite, is a common iron oxide compound with the formula, Fe2O3 and is widely found in rocks and soils. Hematite crystals belong to the rhombohedral lattice system which is designated the alpha polymorph of . It has the same crystal structure as corundum () and ilmenite (). With this it forms a complete solid solution at temperatures above . Hematite naturally occurs in black to steel or silver-gray, brown to reddish-brown, or red colors. It is mined as an important ore mineral of iron. It is electrically conductive. Hematite varieties include ''kidney ore'', ''martite'' (pseudomorphs after magnetite), ''iron rose'' and ''specularite'' (specular hematite). While these forms vary, they all have a rust-red streak. Hematite is not only harder than pure iron, but also much more brittle. Maghemite is a polymorph of hematite (γ-) with the same chemical formula, but with a spinel structure like magnetite. Large deposits of hematite are found in ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |