|
Foams
Foams are materials formed by trapping pockets of gas in a liquid or solid. A bath sponge and the head on a glass of beer are examples of foams. In most foams, the volume of gas is large, with thin films of liquid or solid separating the regions of gas. Soap foams are also known as suds. Solid foams can be closed-cell or open-cell. In closed-cell foam, the gas forms discrete pockets, each completely surrounded by the solid material. In open-cell foam, gas pockets connect to each other. A bath sponge is an example of an open-cell foam: water easily flows through the entire structure, displacing the air. A sleeping mat is an example of a closed-cell foam: gas pockets are sealed from each other so the mat cannot soak up water. Foams are examples of dispersed media. In general, gas is present, so it divides into gas bubbles of different sizes (i.e., the material is polydisperse)—separated by liquid regions that may form films, thinner and thinner when the liquid phase drain ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Foam - Big
Foams are materials science, materials formed by trapping pockets of gas in a liquid or solid. A Sponge (tool), bath sponge and the Beer head, head on a glass of beer are examples of foams. In most foams, the volume of gas is large, with thin films of liquid or solid separating the regions of gas. Soap foams are also known as suds. Solid foams can be closed-cell or reticulated foam, open-cell. In closed-cell foam, the gas forms discrete pockets, each completely surrounded by the solid material. In open-cell foam, gas pockets connect to each other. A bath sponge is an example of an open-cell foam: water easily flows through the entire structure, displacing the air. A sleeping mat is an example of a closed-cell foam: gas pockets are sealed from each other so the mat cannot soak up water. Foams are examples of dispersed media. In general, gas is present, so it divides into gas Bubble (physics), bubbles of different sizes (i.e., the material is polydisperse)—separated by liquid ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Reticulated Foam
Reticulated foam is a very porous, low density solid foam. 'Reticulated' means like a net. Reticulated foams are extremely open foams i.e. there are few, if any, intact bubbles or cell windows. In contrast, the foam formed by soap bubbles is composed solely of intact (fully enclosed) bubbles. In a reticulated foam only the lineal boundaries where the bubbles meet ( Plateau borders) remain. The solid component of a reticulated foam may be an organic polymer like polyurethane, a ceramic or a metal. These materials are used in a wide range of applications where the high porosity and large surface area are needed, including filters, catalyst supports, fuel tank inserts, and loudspeaker covers. Structure and properties A description of the structure of reticulated foams is still being developed. While Plateau's laws, the rules governing the shape of soap films in foams were developed in the 19th century, a mathematical description of the structure is still debated. The computer-genera ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
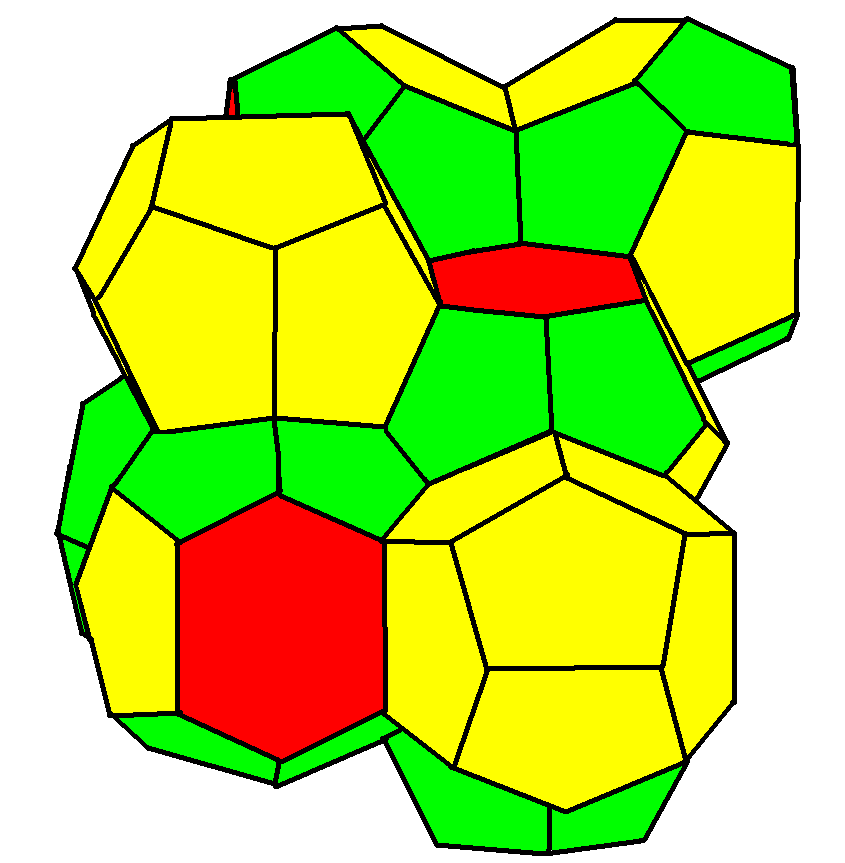
Weaire–Phelan Structure
In geometry, the Weaire–Phelan structure is a three-dimensional structure representing an idealised foam of equal-sized bubbles, with two different shapes. In 1993, Denis Weaire and Robert Phelan found that this structure was a better solution of the Kelvin problem of tiling space by equal volume cells of minimum surface area than the previous best-known solution, the Kelvin structure. History and the Kelvin problem In two dimensions, the subdivision of the plane into cells of equal area with minimum average perimeter is given by the hexagonal tiling, but although the first record of this honeycomb conjecture goes back to the ancient Roman scholar Marcus Terentius Varro, it was not proven until the work of Thomas C. Hales in 1999. In 1887, Lord Kelvin asked the corresponding question for three-dimensional space: how can space be partitioned into cells of equal volume with the least area of surface between them? Or, in short, what was the most efficient soap bubble foam? This ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Tessellations
A tessellation or tiling is the covering of a surface, often a plane (mathematics), plane, using one or more geometric shapes, called ''tiles'', with no overlaps and no gaps. In mathematics, tessellation can be generalized to high-dimensional spaces, higher dimensions and a variety of geometries. A periodic tiling has a repeating pattern. Some special kinds include ''regular tilings'' with regular polygonal tiles all of the same shape, and ''semiregular tilings'' with regular tiles of more than one shape and with every corner identically arranged. The patterns formed by periodic tilings can be categorized into 17 wallpaper groups. A tiling that lacks a repeating pattern is called "non-periodic". An ''aperiodic tiling'' uses a small set of tile shapes that cannot form a repeating pattern. A ''tessellation of space'', also known as a space filling or honeycomb, can be defined in the geometry of higher dimensions. A real physical tessellation is a tiling made of materials such a ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Surfactant
Surfactants are chemical compounds that decrease the surface tension between two liquids, between a gas and a liquid, or interfacial tension between a liquid and a solid. Surfactants may act as detergents, wetting agents, emulsifiers, foaming agents, or dispersants. The word "surfactant" is a blend of ''surface-active agent'', coined . Agents that increase surface tension are "surface active" in the literal sense but are not called surfactants as their effect is opposite to the common meaning. A common example of surface tension increase is salting out: by adding an inorganic salt to an aqueous solution of a weakly polar substance, the substance will precipitate. The substance may itself be a surfactant – this is one of the reasons why many surfactants are ineffective in sea water. Composition and structure Surfactants are usually organic compounds that are amphiphilic, meaning each molecule contains both a hydrophilic "water-seeking" group (the ''head''), and a hydro ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Surfactant
Surfactants are chemical compounds that decrease the surface tension between two liquids, between a gas and a liquid, or interfacial tension between a liquid and a solid. Surfactants may act as detergents, wetting agents, emulsifiers, foaming agents, or dispersants. The word "surfactant" is a blend of ''surface-active agent'', coined . Agents that increase surface tension are "surface active" in the literal sense but are not called surfactants as their effect is opposite to the common meaning. A common example of surface tension increase is salting out: by adding an inorganic salt to an aqueous solution of a weakly polar substance, the substance will precipitate. The substance may itself be a surfactant – this is one of the reasons why many surfactants are ineffective in sea water. Composition and structure Surfactants are usually organic compounds that are amphiphilic, meaning each molecule contains both a hydrophilic "water-seeking" group (the ''head''), and a hydro ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Plateau's Laws
Plateau's laws describe the structure of soap films. These laws were formulated in the 19th century by the Belgian physicist Joseph Plateau from his experimental observations. Many patterns in nature are based on foams obeying these laws. Laws for soap films Plateau's laws describe the shape and configuration of soap films as follows: # Soap films are made of entire (unbroken) smooth surfaces. # The mean curvature of a portion of a soap film is everywhere constant on any point on the same piece of soap film. # Soap films always meet in threes along an edge called a Plateau border, and they do so at an angle of arccos(−) = 120°. # These Plateau borders meet in fours at a vertex, at the tetrahedral angle of arccos(−) ≈ 109.47°. Configurations other than those of Plateau's laws are unstable, and the film will quickly tend to rearrange itself to conform to these laws. That these laws hold for minimal surfaces was proved mathematically by Jean Taylor using geometr ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Colloid
A colloid is a mixture in which one substance consisting of microscopically dispersed insoluble particles is suspended throughout another substance. Some definitions specify that the particles must be dispersed in a liquid, while others extend the definition to include substances like aerosols and gels. The term colloidal suspension refers unambiguously to the overall mixture (although a narrower sense of the word ''suspension'' is distinguished from colloids by larger particle size). A colloid has a dispersed phase (the suspended particles) and a continuous phase (the medium of suspension). The dispersed phase particles have a diameter of approximately 1 nanometre to 1 micrometre. Some colloids are translucent because of the Tyndall effect, which is the scattering of light by particles in the colloid. Other colloids may be opaque or have a slight color. Colloidal suspensions are the subject of interface and colloid science. This field of study was introduced in 1845 by Itali ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Work (physics)
In physics, work is the energy transferred to or from an object via the application of force along a displacement. In its simplest form, for a constant force aligned with the direction of motion, the work equals the product of the force strength and the distance traveled. A force is said to do ''positive work'' if when applied it has a component in the direction of the displacement of the point of application. A force does ''negative work'' if it has a component opposite to the direction of the displacement at the point of application of the force. For example, when a ball is held above the ground and then dropped, the work done by the gravitational force on the ball as it falls is positive, and is equal to the weight of the ball (a force) multiplied by the distance to the ground (a displacement). If the ball is thrown upwards, the work done by its weight is negative, and is equal to the weight multiplied by the displacement in the upwards direction. When the force is consta ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Surface Tension
Surface tension is the tendency of liquid surfaces at rest to shrink into the minimum surface area possible. Surface tension is what allows objects with a higher density than water such as razor blades and insects (e.g. water striders) to float on a water surface without becoming even partly submerged. At liquid–air interfaces, surface tension results from the greater attraction of liquid molecules to each other (due to cohesion) than to the molecules in the air (due to adhesion). There are two primary mechanisms in play. One is an inward force on the surface molecules causing the liquid to contract. Second is a tangential force parallel to the surface of the liquid. This ''tangential'' force is generally referred to as the surface tension. The net effect is the liquid behaves as if its surface were covered with a stretched elastic membrane. But this analogy must not be taken too far as the tension in an elastic membrane is dependent on the amount of deformation of the m ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Pickering Emulsion
A Pickering emulsion is an emulsion that is stabilized by solid particles (for example colloidal silica) which adsorb onto the interface between the water and oil phases. Typically, the emulsions are either water-in-oil or oil-in-water emulsions, but other more complex systems such as water-in-water, oil-in-oil, water-in-oil-in-water, and oil-in-water-in-oil also do exist. Pickering emulsions were named after S.U. Pickering, who described the phenomenon in 1907, although the effect was first recognized by Walter Ramsden in 1903. If oil and water are mixed and small oil droplets are formed and dispersed throughout the water (oil-in-water emulsion), eventually the droplets will coalesce to decrease the amount of energy in the system. However, if solid particles are added to the mixture, they will bind to the surface of the interface and prevent the droplets from coalescing, making the emulsion more stable. Particle properties such as hydrophobicity, shape, and size, as well as the ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Amphiphilic
An amphiphile (from the Greek αμφις amphis, both, and φιλíα philia, love, friendship), or amphipath, is a chemical compound possessing both hydrophilic (''water-loving'', polar) and lipophilic (''fat-loving'') properties. Such a compound is called amphiphilic or amphipathic. Common amphiphilic substances are soaps, detergents, and lipoproteins. The phospholipid amphiphiles are the major structural component of cell membranes. Amphiphiles are the basis for a number of areas of research in chemistry and biochemistry, notably that of lipid polymorphism. Organic compounds containing hydrophilic groups at both ends of the molecule are called bolaamphiphilic. The micelles they form in the aggregate are prolate. Structure The lipophilic group is typically a large hydrocarbon moiety, such as a long chain of the form CH3(CH2)n, with n > 4. The hydrophilic group falls into one of the following categories: # charged groups #* anionic. Examples, with the lipophilic part of the m ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |



.jpg)


