|
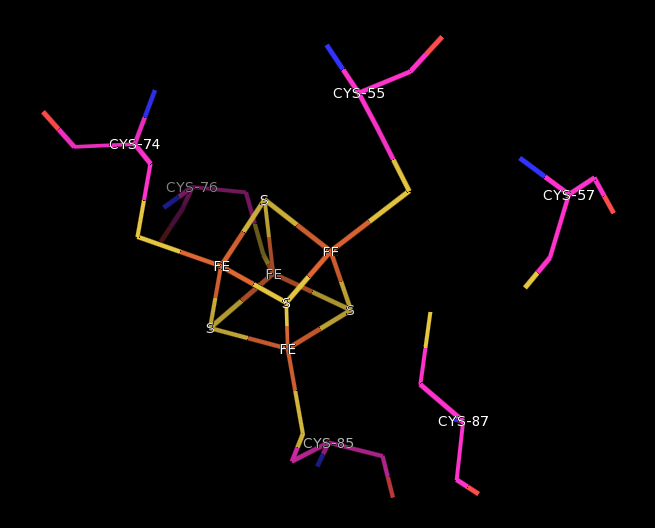
Ferredoxin-thioredoxin Reductase
Ferredoxin-thioredoxin reductase , systematic name ''ferredoxin:thioredoxin disulfide oxidoreductase,'' is a [4Fe-4S] protein that plays an important role in the ferredoxin/thioredoxin regulatory chain. It catalyzes the following reaction: ::: 2 reduced ferredoxin + thioredoxin disulfide \rightleftharpoons 2 oxidized ferredoxin + thioredoxin thiols + 2 H+ Ferredoxin-Thioredoxin reductase (FTR) converts an electron signal (photoreduced ferredoxin) to a thiol signal (reduced thioredoxin), regulating enzymes by Redox, reduction of specific disulfide groups. It catalysis, catalyses the light-dependent activation of several photosynthesis enzymes and constitutes the first historical example of a thiol/disulfide exchange cascade for enzyme regulation. It is a heterodimer of subunit alpha and subunit beta. Subunit alpha is the variable subunit, and beta is the catalytic chain. The secondary structure, structure of the beta subunit has been determined and found to fold around the FeS cl ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Protein
Proteins are large biomolecules and macromolecules that comprise one or more long chains of amino acid residues. Proteins perform a vast array of functions within organisms, including catalysing metabolic reactions, DNA replication, responding to stimuli, providing structure to cells and organisms, and transporting molecules from one location to another. Proteins differ from one another primarily in their sequence of amino acids, which is dictated by the nucleotide sequence of their genes, and which usually results in protein folding into a specific 3D structure that determines its activity. A linear chain of amino acid residues is called a polypeptide. A protein contains at least one long polypeptide. Short polypeptides, containing less than 20–30 residues, are rarely considered to be proteins and are commonly called peptides. The individual amino acid residues are bonded together by peptide bonds and adjacent amino acid residues. The sequence of amino acid residue ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
C4 Carbon Fixation
carbon fixation or the Hatch–Slack pathway is one of three known photosynthetic processes of carbon fixation in plants. It owes the names to the 1960's discovery by Marshall Davidson Hatch and Charles Roger Slack that some plants, when supplied with 14, incorporate the 14C label into four-carbon molecules first. fixation is an addition to the ancestral and more common carbon fixation. The main carboxylating enzyme in photosynthesis is called RuBisCO, which catalyses two distinct reactions using either (carboxylation) or oxygen (oxygenation) as a substrate. The latter process, oxygenation, gives rise to the wasteful process of photorespiration. photosynthesis reduces photorespiration by concentrating around RuBisCO. To ensure that RuBisCO works in an environment where there is a lot of carbon dioxide and very little oxygen, leaves generally differentiate two partially isolated compartments called mesophyll cells and bundle-sheath cells. is initially fixed in the ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Protein Dimer
In biochemistry, a protein dimer is a macromolecular complex formed by two protein monomers, or single proteins, which are usually non-covalently bound. Many macromolecules, such as proteins or nucleic acids, form dimers. The word ''dimer'' has roots meaning "two parts", '' di-'' + '' -mer''. A protein dimer is a type of protein quaternary structure. A protein homodimer is formed by two identical proteins. A protein heterodimer is formed by two different proteins. Most protein dimers in biochemistry are not connected by covalent bonds. An example of a non-covalent heterodimer is the enzyme reverse transcriptase, which is composed of two different amino acid chains. An exception is dimers that are linked by disulfide bridges such as the homodimeric protein NEMO. Some proteins contain specialized domains to ensure dimerization (dimerization domains) and specificity. The G protein-coupled cannabinoid receptors have the ability to form both homo- and heterodimers with several ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Phosphoribulokinase
Phosphoribulokinase (PRK) () is an essential photosynthetic enzyme that catalyzes the ATP-dependent phosphorylation of ribulose 5-phosphate (RuP) into ribulose 1,5-bisphosphate (RuBP), both intermediates in the Calvin Cycle. Its main function is to regenerate RuBP, which is the initial substrate and CO2-acceptor molecule of the Calvin Cycle. PRK belongs to the family of transferase enzymes, specifically those transferring phosphorus-containing groups (phosphotransferases) to an alcohol group acceptor. Along with ribulose 1,5-bisphosphate carboxylase/oxygenase (RuBisCo), phosphoribulokinase is unique to the Calvin Cycle. Therefore, PRK activity often determines the metabolic rate in organisms for which carbon fixation is key to survival. Much initial work on PRK was done with spinach leaf extracts in the 1950s; subsequent studies of PRK in other photosynthetic prokaryotic and eukaryotic organisms have followed. The possibility that PRK might exist was first recognized by Weissbach ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Sedoheptulose-bisphosphatase
Sedoheptulose-bisphosphatase (also sedoheptulose-1,7-bisphosphatase or SBPase, EC number 3.1.3.37; systematic name sedoheptulose-1,7-bisphosphate 1-phosphohydrolase) is an enzyme that catalyzes the removal of a phosphate group from sedoheptulose 1,7-bisphosphate to produce sedoheptulose 7-phosphate. SBPase is an example of a phosphatase, or, more generally, a hydrolase. This enzyme participates in the Calvin cycle. Structure SBPase is a homodimeric protein, meaning that it is made up of two identical subunits. The size of this protein varies between species, but is about 92,000 Atomic mass unit, Da (two 46,000 Da subunits) in cucumber plant leaves. The key functional domain controlling SBPase function involves a disulfide bond between two cysteine residues. These two cysteine residues, Cys52 and Cys57, appear to be located in a flexible loop between the two subunits of the homodimer, near the active site of the enzyme. Reduction of this regulatory disulfide bond by thioredoxin i ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Fructose-1,6-bisphosphatase
The enzyme fructose bisphosphatase (EC 3.1.3.11; systematic name D-fructose-1,6-bisphosphate 1-phosphohydrolase) catalyses the conversion of fructose-1,6-bisphosphate to fructose 6-phosphate in gluconeogenesis and the Calvin cycle, which are both anabolic pathways: :D-fructose 1,6-bisphosphate + H2O = D-fructose 6-phosphate + phosphate Phosphofructokinase (EC 2.7.1.11) catalyses the reverse conversion of fructose 6-phosphate to fructose-1,6-bisphosphate, but this is not just the reverse reaction, because the co-substrates are different (and so thermodynamic requirements are not violated). The two enzymes each catalyse the conversion in one direction only, and are regulated by metabolites such as fructose 2,6-bisphosphate so that high activity of one of them is accompanied by low activity of the other. More specifically, fructose 2,6-bisphosphate allosterically inhibits fructose 1,6-bisphosphatase, but activates phosphofructokinase-I. Fructose 1,6-bisphosphatase is involved ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Disulfide Bond
In biochemistry, a disulfide (or disulphide in British English) refers to a functional group with the structure . The linkage is also called an SS-bond or sometimes a disulfide bridge and is usually derived by the coupling of two thiol groups. In biology, disulfide bridges formed between thiol groups in two cysteine residues are an important component of the secondary and tertiary structure of proteins. ''Persulfide'' usually refers to compounds. In inorganic chemistry disulfide usually refers to the corresponding anion (−S−S−). Organic disulfides Symmetrical disulfides are compounds of the formula . Most disulfides encountered in organo sulfur chemistry are symmetrical disulfides. Unsymmetrical disulfides (also called heterodisulfides) are compounds of the formula . They are less common in organic chemistry, but most disulfides in nature are unsymmetrical. Properties The disulfide bonds are strong, with a typical bond dissociation energy of 60 kcal/mol (251& ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Thioredoxin
Thioredoxin is a class of small redox proteins known to be present in all organisms. It plays a role in many important biological processes, including redox signaling. In humans, thioredoxins are encoded by ''TXN'' and ''TXN2'' genes. Loss-of-function mutation of either of the two human thioredoxin genes is lethal at the four-cell stage of the developing embryo. Although not entirely understood, thioredoxin is linked to medicine through their response to reactive oxygen species (ROS). In plants, thioredoxins regulate a spectrum of critical functions, ranging from photosynthesis to growth, flowering and the development and germination of seeds. Thioredoxins play a role in cell-to-cell communication. Occurrence They are found in nearly all known organisms and are essential for life in mammals. Function The primary function of Thioredoxin (Trx) is the reduction of oxidized cysteine residues and the cleavage of disulfide bonds. Multiple in vitro substrates for thioredoxin have be ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Ferredoxin
Ferredoxins (from Latin ''ferrum'': iron + redox, often abbreviated "fd") are iron–sulfur proteins that mediate electron transfer in a range of metabolic reactions. The term "ferredoxin" was coined by D.C. Wharton of the DuPont Co. and applied to the "iron protein" first purified in 1962 by Mortenson, Valentine, and Carnahan from the anaerobic bacterium '' Clostridium pasteurianum''. Another redox protein, isolated from spinach chloroplasts, was termed "chloroplast ferredoxin". The chloroplast ferredoxin is involved in both cyclic and non-cyclic photophosphorylation reactions of photosynthesis. In non-cyclic photophosphorylation, ferredoxin is the last electron acceptor thus reducing the enzyme NADP+ reductase. It accepts electrons produced from sunlight- excited chlorophyll and transfers them to the enzyme ferredoxin: NADP+ oxidoreductase . Ferredoxins are small proteins containing iron and sulfur atoms organized as iron–sulfur clusters. These biological " capacitors" can ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Organic Redox Reaction
Organic reductions or organic oxidations or organic redox reactions are redox reactions that take place with organic compounds. In organic chemistry oxidations and reductions are different from ordinary redox reactions, because many reactions carry the name but do not actually involve electron transfer.March Jerry; (1985). Advanced Organic Chemistry reactions, mechanisms and structure (3rd ed.). New York: John Wiley & Sons, inc. Instead the relevant criterion for organic oxidation is gain of oxygen and/or loss of hydrogen, respectively.''Organic Redox Systems: Synthesis, Properties, and Applications'', Tohru Nishinaga 2016 Simple functional groups can be arranged in order of increasing oxidation state. The oxidation numbers are only an approximation: When methane is oxidized to carbon dioxide its oxidation number changes from −4 to +4. Classical reductions include alkene reduction to alkanes and classical oxidations include oxidation of alcohols to aldehydes. In oxidations ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Pentose Phosphate Pathway
The pentose phosphate pathway (also called the phosphogluconate pathway and the hexose monophosphate shunt and the HMP Shunt) is a metabolic pathway parallel to glycolysis. It generates NADPH and pentoses (5-carbon sugars) as well as ribose 5-phosphate, a precursor for the synthesis of nucleotides. While the pentose phosphate pathway does involve oxidation of glucose, its primary role is anabolic rather than catabolic. The pathway is especially important in red blood cells (erythrocytes). There are two distinct phases in the pathway. The first is the oxidative phase, in which NADPH is generated, and the second is the non-oxidative synthesis of 5-carbon sugars. For most organisms, the pentose phosphate pathway takes place in the cytosol; in plants, most steps take place in plastids. Like glycolysis, the pentose phosphate pathway appears to have a very ancient evolutionary origin. The reactions of this pathway are mostly enzyme-catalyzed in modern cells, however, they also occur ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Calvin Cycle
The Calvin cycle, light-independent reactions, bio synthetic phase, dark reactions, or photosynthetic carbon reduction (PCR) cycle of photosynthesis is a series of chemical reactions that convert carbon dioxide and hydrogen-carrier compounds into glucose. The Calvin cycle is present in all photosynthetic eukaryotes and also many photosynthetic bacteria. In plants, these reactions occur in the stroma, the fluid-filled region of a chloroplast outside the thylakoid membranes. These reactions take the products ( ATP and NADPH) of light-dependent reactions and perform further chemical processes on them. The Calvin cycle uses the chemical energy of ATP and reducing power of NADPH from the light dependent reactions to produce sugars for the plant to use. These substrates are used in a series of reduction-oxidation reactions to produce sugars in a step-wise process; there is no direct reaction that converts several molecules of to a sugar. There are three phases to the light-independ ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |