|
Esperamicin
The esperamicins are chromoprotein enediyne antitumor antibiotics of bacterial origin. Esperamicin A1 is the most well studied compound in this class. Esperamcin A1 and the related enediyne calicheamicin are the two most potent antitumor agents known. The esperamicins are extremely toxic DNA splicing compounds. Oxygen and active oxygen-radical scavengers have no significant influence upon DNA strand breakage by esperamicin, but the cleavage of DNA by esperamicin is greatly accelerated in the presence of thiol compounds. The preferential cutting sites of esperamicin are at thymidylate residues, and the frequency of nucleobase attacked (T greater than C greater than A greater than G) is different from that of calicheamicin (C much greater than T greater than A = G), neocarzinostatin (T greater than A greater than C greater than G), or bleomycin -13- (1''H''-imidazol-5-yl)methyl9-hydroxy-5- 1''R'')-1-hydroxyethyl8,10-dimethyl-4,7,12,15-tetraoxo-3,6,11,14-tetraazapentadec-1-yl ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
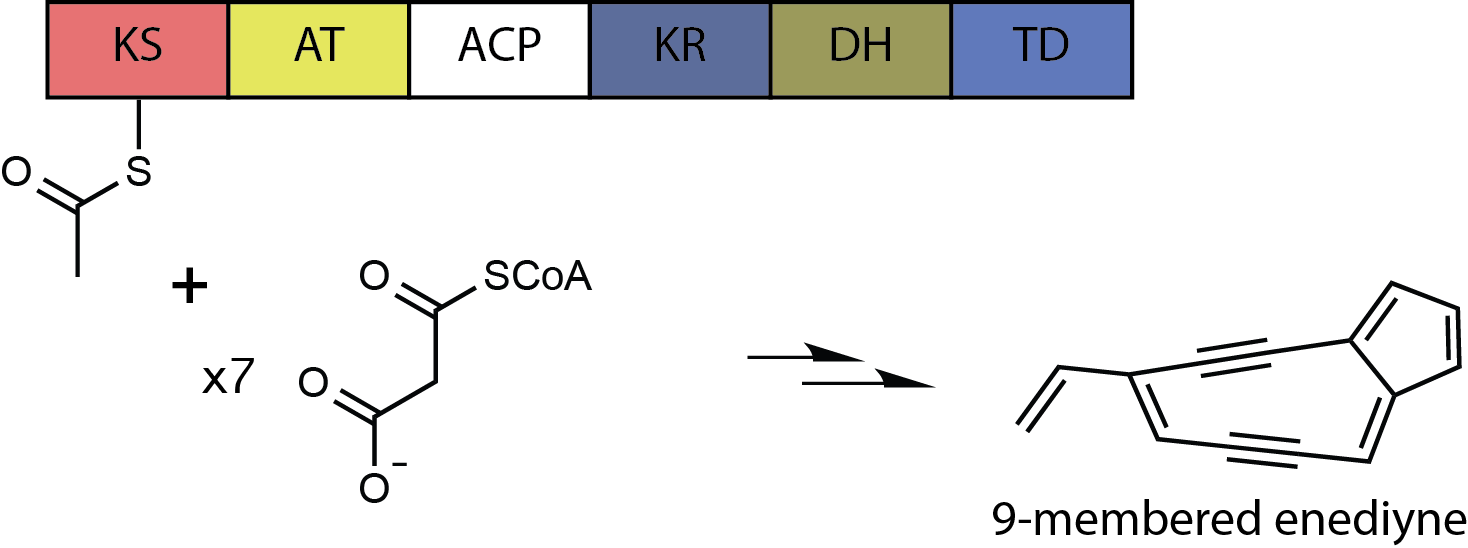
Enediyne
In organic chemistry, enediynes are organic compounds containing two triple bonds and one double bond. Enediynes are most notable for their limited use as antitumor antibiotics (known as enediyne Chemotherapy, anticancer antibiotics). They are efficient at inducing apoptosis in Cell biology, cells, but cannot differentiate Cancer, cancerous cells from healthy cells. Consequently, research is being conducted to increase the specificity of enediyne toxicity. Structure and reactivity A nine- or ten-membered ring containing a double bond between two triple bonds is termed the warhead of the enediyne. In this state, the warhead is inactive. Enediynes are triggered into a chemically active state via Bergman cyclization, Bergman or Myers-Saito cyclization. The triggering mechanism can be attributed to an intramolecular nucleophilic attack initiated by one of the variable regions of the molecule. Triggering can also occur via attack by an external nucleophile. Bergman cyclization re ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Enediynes
In organic chemistry, enediynes are organic compounds containing two triple bonds and one double bond. Enediynes are most notable for their limited use as antitumor antibiotics (known as enediyne anticancer antibiotics). They are efficient at inducing apoptosis in cells, but cannot differentiate cancerous cells from healthy cells. Consequently, research is being conducted to increase the specificity of enediyne toxicity. Structure and reactivity A nine- or ten-membered ring containing a double bond between two triple bonds is termed the warhead of the enediyne. In this state, the warhead is inactive. Enediynes are triggered into a chemically active state via Bergman or Myers-Saito cyclization. The triggering mechanism can be attributed to an intramolecular nucleophilic attack initiated by one of the variable regions of the molecule. Triggering can also occur via attack by an external nucleophile. Bergman cyclization restructures the enediyne ring into two smaller rings. O ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Calicheamicin
The calicheamicins are a class of enediyne antitumor antibiotics derived from the bacterium ''Micromonospora echinospora'', with calicheamicin γ1 being the most notable. It was isolated originally in the mid-1980s from the chalky soil, or "caliche pits", located in Kerrville, Texas. The sample was collected by a scientist working for Lederle Labs. It is extremely toxic to all cells and, in 2000, a CD33 antigen-targeted immunoconjugate N-acetyl dimethyl hydrazide calicheamicin was developed and marketed as targeted therapy against the non-solid tumor cancer acute myeloid leukemia (AML). A second calicheamicin-linked monoclonal antibody, inotuzumab ozogamicin (marketed as Besponsa) an anti-CD22-directed antibody-drug conjugate, was approved by the U.S. Food and Drug Administration on August 17, 2017, for use in the treatment of adults with relapsed or refractory B-cell precursor acute lymphoblastic leukemia. Calicheamicin γ1 and the related enediyne esperamicin are the two of the m ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Chromoprotein
A chromoprotein is a conjugated protein that contains a pigmented prosthetic group (or cofactor). A common example is haemoglobin, which contains a heme cofactor, which is the iron-containing molecule that makes oxygenated blood appear red. Other examples of chromoproteins include other hemochromes, cytochromes, phytochromes and flavoproteins. In hemoglobin there exists a chromoprotein (tetramer MW:4 x 16.125 =64.500), namely heme, consisting of Fe++ four pyrrol rings. A single chromoprotein can act as both a phytochrome and a phototropin due to the presence and processing of multiple chromophores. Phytochrome in ferns contains PHY3 which contains an unusual photoreceptor with a dual-channel possessing both phytochrome (red-light sensing) and phototropin (blue-light sensing) and this helps the growth of fern plants at low sunlight. The GFP protein family includes both fluorescent proteins and non-fluorescent chromoproteins. Through mutagenesis or irradiation, the non-fluorescent ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Secondary Alcohols
In chemistry, an alcohol is a type of organic compound that carries at least one hydroxyl () functional group bound to a saturated carbon atom. The term ''alcohol'' originally referred to the primary alcohol ethanol (ethyl alcohol), which is used as a drug and is the main alcohol present in alcoholic drinks. An important class of alcohols, of which methanol and ethanol are the simplest examples, includes all compounds which conform to the general formula . Simple monoalcohols that are the subject of this article include primary (), secondary () and tertiary () alcohols. The suffix ''-ol'' appears in the IUPAC chemical name of all substances where the hydroxyl group is the functional group with the highest priority. When a higher priority group is present in the compound, the prefix ''hydroxy-'' is used in its IUPAC name. The suffix ''-ol'' in non-IUPAC names (such as paracetamol or cholesterol) also typically indicates that the substance is an alcohol. However, some compound ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Methyl Esters
In organic chemistry, a methyl group is an alkyl derived from methane, containing one carbon atom bonded to three hydrogen atoms, having chemical formula . In formulas, the group is often abbreviated as Me. This hydrocarbon group occurs in many organic compounds. It is a very stable group in most molecules. While the methyl group is usually part of a larger molecule, bounded to the rest of the molecule by a single covalent bond (), it can be found on its own in any of three forms: methanide anion (), methylium cation () or methyl radical (). The anion has eight valence electrons, the radical seven and the cation six. All three forms are highly reactive and rarely observed. Methyl cation, anion, and radical Methyl cation The methylium cation () exists in the gas phase, but is otherwise not encountered. Some compounds are considered to be sources of the cation, and this simplification is used pervasively in organic chemistry. For example, protonation of methanol gives an electr ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Benzoate Esters
Benzoic acid is a white (or colorless) solid organic compound with the formula , whose structure consists of a benzene ring () with a carboxyl () substituent. It is the simplest aromatic carboxylic acid. The name is derived from gum benzoin, which was for a long time its only source. Benzoic acid occurs naturally in many plants and serves as an intermediate in the biosynthesis of many secondary metabolites. Salts of benzoic acid are used as food preservatives. Benzoic acid is an important precursor for the industrial synthesis of many other organic substances. The salts and esters of benzoic acid are known as benzoates . History Benzoic acid was discovered in the sixteenth century. The dry distillation of gum benzoin was first described by Nostradamus (1556), and then by Alexius Pedemontanus (1560) and Blaise de Vigenère (1596). Justus von Liebig and Friedrich Wöhler determined the composition of benzoic acid. These latter also investigated how hippuric acid is related ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Anilides
Anilides (or phenylamides) are a class of chemical compounds, which are amide derivatives of aniline. Preparation Aniline reacts with acyl chlorides or carboxylic anhydrides to give anilides. For example, reaction of aniline with acetyl chloride provides acetanilide (CH3-CO-NH-C6H5). At high temperatures, aniline and carboxylic acids react to give anilides. Uses * Herbicides * Fungicides - Oxycarboxin, Carboxin {{Short pages monitor [Baidu] |
Alkene Derivatives
In organic chemistry, an alkene is a hydrocarbon containing a carbon–carbon double bond. Alkene is often used as synonym of olefin, that is, any hydrocarbon containing one or more double bonds.H. Stephen Stoker (2015): General, Organic, and Biological Chemistry'. 1232 pages. Two general types of monoalkenes are distinguished: terminal and internal. Also called α-olefins, terminal alkenes are more useful. However, the International Union of Pure and Applied Chemistry (IUPAC) recommends using the name "alkene" only for acyclic hydrocarbons with just one double bond; alkadiene, alkatriene, etc., or polyene for acyclic hydrocarbons with two or more double bonds; cycloalkene, cycloalkadiene, etc. for cyclic ones; and "olefin" for the general class – cyclic or acyclic, with one or more double bonds. Acyclic alkenes, with only one double bond and no other functional groups (also known as mono-enes) form a homologous series of hydrocarbons with the general formula with '' ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Thioethers
In organic chemistry, an organic sulfide (British English sulphide) or thioether is an organosulfur functional group with the connectivity as shown on right. Like many other sulfur-containing compounds, volatile sulfides have foul odors. A sulfide is similar to an ether except that it contains a sulfur atom in place of the oxygen. The grouping of oxygen and sulfur in the periodic table suggests that the chemical properties of ethers and sulfides are somewhat similar, though the extent to which this is true in practice varies depending on the application. Nomenclature Sulfides are sometimes called thioethers, especially in the old literature. The two organic substituents are indicated by the prefixes. (CH3)2S is called dimethylsulfide. Some sulfides are named by modifying the common name for the corresponding ether. For example, C6H5SCH3 is methyl phenyl sulfide, but is more commonly called thioanisole, since its structure is related to that for anisole, C6H5OCH3. The modern s ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Ethers
In organic chemistry, ethers are a class of compounds that contain an ether group—an oxygen atom connected to two alkyl or aryl groups. They have the general formula , where R and R′ represent the alkyl or aryl groups. Ethers can again be classified into two varieties: if the alkyl or aryl groups are the same on both sides of the oxygen atom, then it is a simple or symmetrical ether, whereas if they are different, the ethers are called mixed or unsymmetrical ethers. A typical example of the first group is the solvent and anaesthetic diethyl ether, commonly referred to simply as "ether" (). Ethers are common in organic chemistry and even more prevalent in biochemistry, as they are common linkages in carbohydrates and lignin. Structure and bonding Ethers feature bent C–O–C linkages. In dimethyl ether, the bond angle is 111° and C–O distances are 141 pm. The barrier to rotation about the C–O bonds is low. The bonding of oxygen in ethers, alcohols, and water is ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Phenol Ethers
Phenol (also called carbolic acid) is an aromatic organic compound with the molecular formula . It is a white crystalline solid that is volatile. The molecule consists of a phenyl group () bonded to a hydroxy group (). Mildly acidic, it requires careful handling because it can cause chemical burns. Phenol was first extracted from coal tar, but today is produced on a large scale (about 7 billion kg/year) from petroleum-derived feedstocks. It is an important industrial commodity as a precursor to many materials and useful compounds. It is primarily used to synthesize plastics and related materials. Phenol and its chemical derivatives are essential for production of polycarbonates, epoxies, Bakelite, nylon, detergents, herbicides such as phenoxy herbicides, and numerous pharmaceutical drugs. Properties Phenol is an organic compound appreciably soluble in water, with about 84.2 g dissolving in 1000 mL (0.895 M). Homogeneous mixtures of phenol and water at phenol to ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |