|
Chromoprotein
A chromoprotein is a conjugated protein that contains a pigmented prosthetic group (or cofactor). A common example is haemoglobin, which contains a heme cofactor, which is the iron-containing molecule that makes oxygenated blood appear red. Other examples of chromoproteins include other hemochromes, cytochromes, phytochromes and flavoproteins. In hemoglobin there exists a chromoprotein (tetramer MW:4 x 16.125 =64.500), namely heme, consisting of Fe++ four pyrrol rings. A single chromoprotein can act as both a phytochrome and a phototropin due to the presence and processing of multiple chromophores. Phytochrome in ferns contains PHY3 which contains an unusual photoreceptor with a dual-channel possessing both phytochrome (red-light sensing) and phototropin (blue-light sensing) and this helps the growth of fern plants at low sunlight. The GFP protein family includes both fluorescent proteins and non-fluorescent chromoproteins. Through mutagenesis or irradiation, the non-fluorescent ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Pigment
A pigment is a colored material that is completely or nearly insoluble in water. In contrast, dyes are typically soluble, at least at some stage in their use. Generally dyes are often organic compounds whereas pigments are often inorganic compounds. Pigments of prehistoric and historic value include ochre, charcoal, and lapis lazuli. Economic impact In 2006, around 7.4 million tons of inorganic, organic, and special pigments were marketed worldwide. Estimated at around US$14.86 billion in 2018 and will rise at over 4.9% CAGR from 2019 to 2026. The global demand for pigments was roughly US$20.5 billion in 2009. According to an April 2018 report by ''Bloomberg Businessweek'', the estimated value of the pigment industry globally is $30 billion. The value of titanium dioxide – used to enhance the white brightness of many products – was placed at $13.2 billion per year, while the color Ferrari red is valued at $300 million each year. Physical principles ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
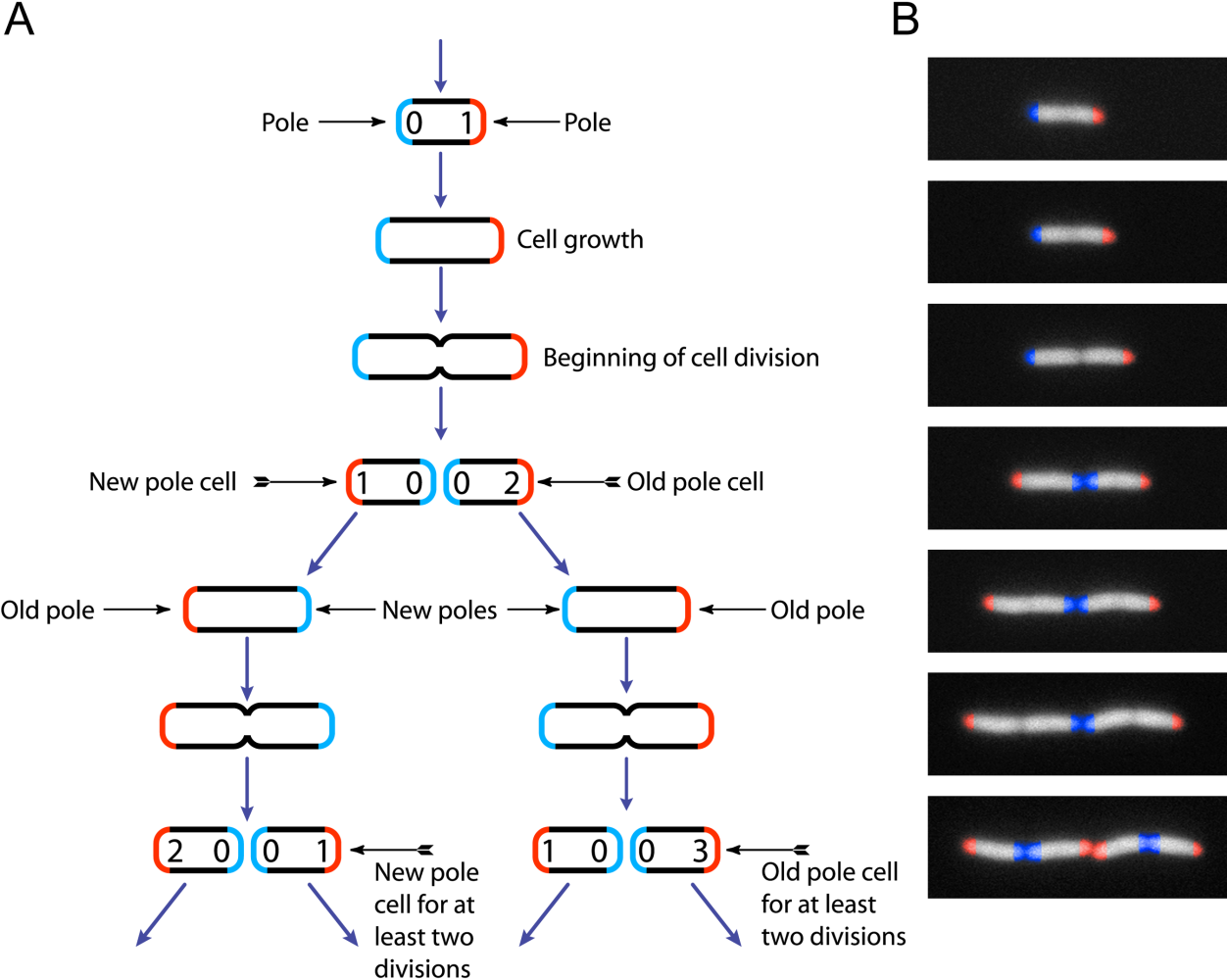
GFP Protein
The green fluorescent protein (GFP) is a protein that exhibits bright green fluorescence when exposed to light in the blue to ultraviolet range. The label ''GFP'' traditionally refers to the protein first isolated from the jellyfish ''Aequorea victoria'' and is sometimes called ''avGFP''. However, GFPs have been found in other organisms including corals, sea anemones, zoanithids, copepods and lancelets. The GFP from ''A. victoria'' has a major excitation peak at a wavelength of 395 nm and a minor one at 475 nm. Its emission peak is at 509 nm, which is in the lower green portion of the visible spectrum. The fluorescence quantum yield (QY) of GFP is 0.79. The GFP from the sea pansy ('' Renilla reniformis'') has a single major excitation peak at 498 nm. GFP makes for an excellent tool in many forms of biology due to its ability to form an internal chromophore without requiring any accessory cofactors, gene products, or enzymes / substrates other than m ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Red Fluorescent Protein
Red fluorescent protein (RFP) is a fluorophore that fluoresces red-orange when excited. Several variants have been developed using directed mutagenesis. The original was isolated from Discosoma, and named DsRed. Others are now available that fluoresce orange, red, and far-red. RFP is approximately 25.9 kDa. The excitation maximum is 558 nm, and the emission maximum is 583 nm. The first fluorescent protein to be discovered, green fluorescent protein (GFP), has been adapted to identify and develop fluorescent markers in other colors. Variants such as yellow fluorescent protein (YFP) and cyan fluorescent protein (CFP) were discovered in Anthozoa. Issues with fluorescent proteins include the length of time between protein synthesis and expression of fluorescence. DsRed has an maturation time of around 24 hours, which can make it unusable for many experiments that take place in a shorter time frame. Additionally, DsRed exists in a tetrameric form, which can affect the functi ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Synthetic Biology
Synthetic biology (SynBio) is a multidisciplinary area of research that seeks to create new biological parts, devices, and systems, or to redesign systems that are already found in nature. It is a branch of science that encompasses a broad range of methodologies from various disciplines, such as biotechnology, biomaterials, material science/engineering, genetic engineering, molecular biology, molecular engineering, systems biology, membrane science, biophysics, chemical and biological engineering, electrical and computer engineering, control engineering and evolutionary biology. Due to more powerful genetic engineering capabilities and decreased DNA synthesis and sequencing costs, the field of synthetic biology is rapidly growing. In 2016, more than 350 companies across 40 countries were actively engaged in synthetic biology applications; all these companies had an estimated net worth of $3.9 billion in the global market. Definition Synthetic biology currently has no gen ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Escherichia Coli
''Escherichia coli'' (),Wells, J. C. (2000) Longman Pronunciation Dictionary. Harlow ngland Pearson Education Ltd. also known as ''E. coli'' (), is a Gram-negative, facultative anaerobic, rod-shaped, coliform bacterium of the genus ''Escherichia'' that is commonly found in the lower intestine of warm-blooded organisms. Most ''E. coli'' strains are harmless, but some serotypes ( EPEC, ETEC etc.) can cause serious food poisoning in their hosts, and are occasionally responsible for food contamination incidents that prompt product recalls. Most strains do not cause disease in humans and are part of the normal microbiota of the gut; such strains are harmless or even beneficial to humans (although these strains tend to be less studied than the pathogenic ones). For example, some strains of ''E. coli'' benefit their hosts by producing vitamin K2 or by preventing the colonization of the intestine by pathogenic bacteria. These mutually beneficial relationships between ''E. col ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Absorption Spectroscopy
Absorption spectroscopy refers to spectroscopic techniques that measure the absorption of radiation, as a function of frequency or wavelength, due to its interaction with a sample. The sample absorbs energy, i.e., photons, from the radiating field. The intensity of the absorption varies as a function of frequency, and this variation is the absorption spectrum. Absorption spectroscopy is performed across the electromagnetic spectrum. Absorption spectroscopy is employed as an analytical chemistry tool to determine the presence of a particular substance in a sample and, in many cases, to quantify the amount of the substance present. Infrared and ultraviolet–visible spectroscopy are particularly common in analytical applications. Absorption spectroscopy is also employed in studies of molecular and atomic physics, astronomical spectroscopy and remote sensing. There is a wide range of experimental approaches for measuring absorption spectra. The most common arrangement is to direct ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Imidazolidine
Imidazolidine is a heterocyclic compound (CH2)2(NH)2CH2. The parent imidazolidine is lightly studied, but related compounds substituted on one or both nitrogen centers are more common. Generally, they are colorless, polar, basic compounds. Imidazolidines are cyclic 5-membered examples of the general class of aminals. Preparation Imidazolidines are traditionally prepared by condensation reaction of 1,2-diamines and aldehydes. Most commonly, one or both nitrogen center is substituted with an alkyl or benzyl (Bn) group:Ferm, R. J.; Riebsomer, J. L. From "The chemistry of the 2-imidazolines and imidazolidines" Chemical Reviews, 1954, 54, 593-613. :(CH2NBn)2 + PhCHO → (CH2NBn)2C(H)Ph + H2O The first unsubstituted imidazolidine synthesis was reported in 1952. Reactions Unsubstituted imidazolidines are often labile. The rings are susceptible to hydrolysis back to the diamine and the aldehyde. Formally, removal of the two hydrogens at carbon 2 (between the two nitrogens) w ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Conjugated System
In theoretical chemistry, a conjugated system is a system of connected p-orbitals with delocalized electrons in a molecule, which in general lowers the overall energy of the molecule and increases stability. It is conventionally represented as having alternating single and multiple bonds. Lone pairs, radicals or carbenium ions may be part of the system, which may be cyclic, acyclic, linear or mixed. The term "conjugated" was coined in 1899 by the German chemist Johannes Thiele. Conjugation is the overlap of one p-orbital with another across an adjacent σ bond (in transition metals, d-orbitals can be involved). A conjugated system has a region of overlapping p-orbitals, bridging the interjacent locations that simple diagrams illustrate as not having a π bond. They allow a delocalization of π electrons across all the adjacent aligned p-orbitals. The π electrons do not belong to a single bond or atom, but rather to a group of atoms. Molecules containing conjugated syst ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Phenols
In organic chemistry, phenols, sometimes called phenolics, are a class of chemical compounds consisting of one or more hydroxyl groups (— O H) bonded directly to an aromatic hydrocarbon group. The simplest is phenol, . Phenolic compounds are classified as simple phenols or polyphenols based on the number of phenol units in the molecule. Phenols are both synthesized industrially and produced by plants and microorganisms. Properties Acidity Phenols are more acidic than typical alcohols. The acidity of the hydroxyl group in phenols is commonly intermediate between that of aliphatic alcohols and carboxylic acids (their pKa is usually between 10 and 12). Deprotonation of a phenol forms a corresponding negative phenolate ion or phenoxide ion, and the corresponding salts are called phenolates or phenoxides (aryloxides according to the IUPAC Gold Book). Condensation with aldehydes and ketones Phenols are susceptible to Electrophilic aromatic substitutions. Condensation with formald ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Anemonia Sulcata
''Anemonia sulcata'', or Mediterranean snakelocks sea anemone, is a species of sea anemone in the family Actiniidae from the Mediterranean Sea. Whether ''A. sulcata'' should be recognized as a synonym of '' A. viridis'' remains a matter of dispute. Description This sea anemone has two ecotypes; one has a basal disk up to in diameter and has fewer than 192 tentacles (usually 142 to 148); the other has a disk up to in diameter and 192 tentacles or more, up to 348. The tentacles are long, slender and tapering, arranged in six whorls round the central mouth on the oral disk. They vary in colour but are usually some shade of green, grey or light brown. A knob on the tip of each tentacle, where the stinging cells are concentrated, may be violet. Distribution and habitat This sea anemone is native to the Mediterranean Sea and the eastern Atlantic Ocean as far south as Western Sahara. It is found in the intertidal zone and the sublittoral zone, on rocky ledges, in crevices and on b ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Phototropin
Phototropins are photoreceptor proteins (more specifically, flavoproteins) that mediate phototropism responses in higher plants. Phototropins can be found throughout the leaves of a plant. Along with cryptochromes and phytochromes they allow plants to respond and alter their growth in response to the light environment. Phototropins may also be important for the opening of stomata and the movement of chloroplasts. These blue light receptors are seen across the entire green plant lineage. When Phototropins are hit with blue light, they induce a signal transduction pathway that alters the plant cells' functions in different ways. Phototropins are part of the phototropic sensory system in plants that causes various environmental responses in plants. Phototropins specifically will cause stems to bend towards light and stomata to open. Phototropins have been shown to impact the movement of chloroplast inside the cell. In addition phototropins mediate the first changes in stem elongation ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |