|
Disjoining Pressure
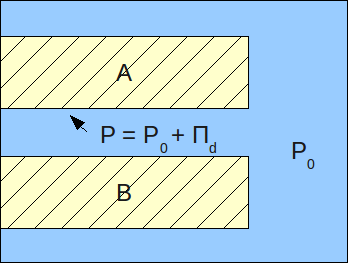
In surface chemistry, disjoining pressure (symbol ) according to an IUPAC definition arises from an attractive interaction between two surfaces. For two flat and parallel surfaces, the value of the disjoining pressure (i.e., the force per unit area) can be calculated as the derivative of the Gibbs energy of interaction per unit area in respect to distance (in the direction normal to that of the interacting surfaces). There is also a related concept of disjoining force, which can be viewed as disjoining pressure times the surface area of the interacting surfaces. The concept of disjoining pressure was introduced by Derjaguin (1936) as the difference between the pressure in a region of a phase adjacent to a surface confining it, and the pressure in the bulk of this phase. Description Disjoining pressure can be expressed as:Hans-Jürgen Butt, Karlheinz Graf, Michael Kappl,"Physics and chemistry of interfaces", John Wiley & Sons Canada, Ltd., 1 edition, 2003, page 9(Google book ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Surface Chemistry
Surface science is the study of physical and chemical phenomena that occur at the interface of two phases, including solid–liquid interfaces, solid– gas interfaces, solid–vacuum interfaces, and liquid– gas interfaces. It includes the fields of '' surface chemistry'' and ''surface physics''. Some related practical applications are classed as surface engineering. The science encompasses concepts such as heterogeneous catalysis, semiconductor device fabrication, fuel cells, self-assembled monolayers, and adhesives. Surface science is closely related to interface and colloid science. Interfacial chemistry and physics are common subjects for both. The methods are different. In addition, interface and colloid science studies macroscopic phenomena that occur in heterogeneous systems due to peculiarities of interfaces. History The field of surface chemistry started with heterogeneous catalysis pioneered by Paul Sabatier on hydrogenation and Fritz Haber on the Haber process. Ir ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
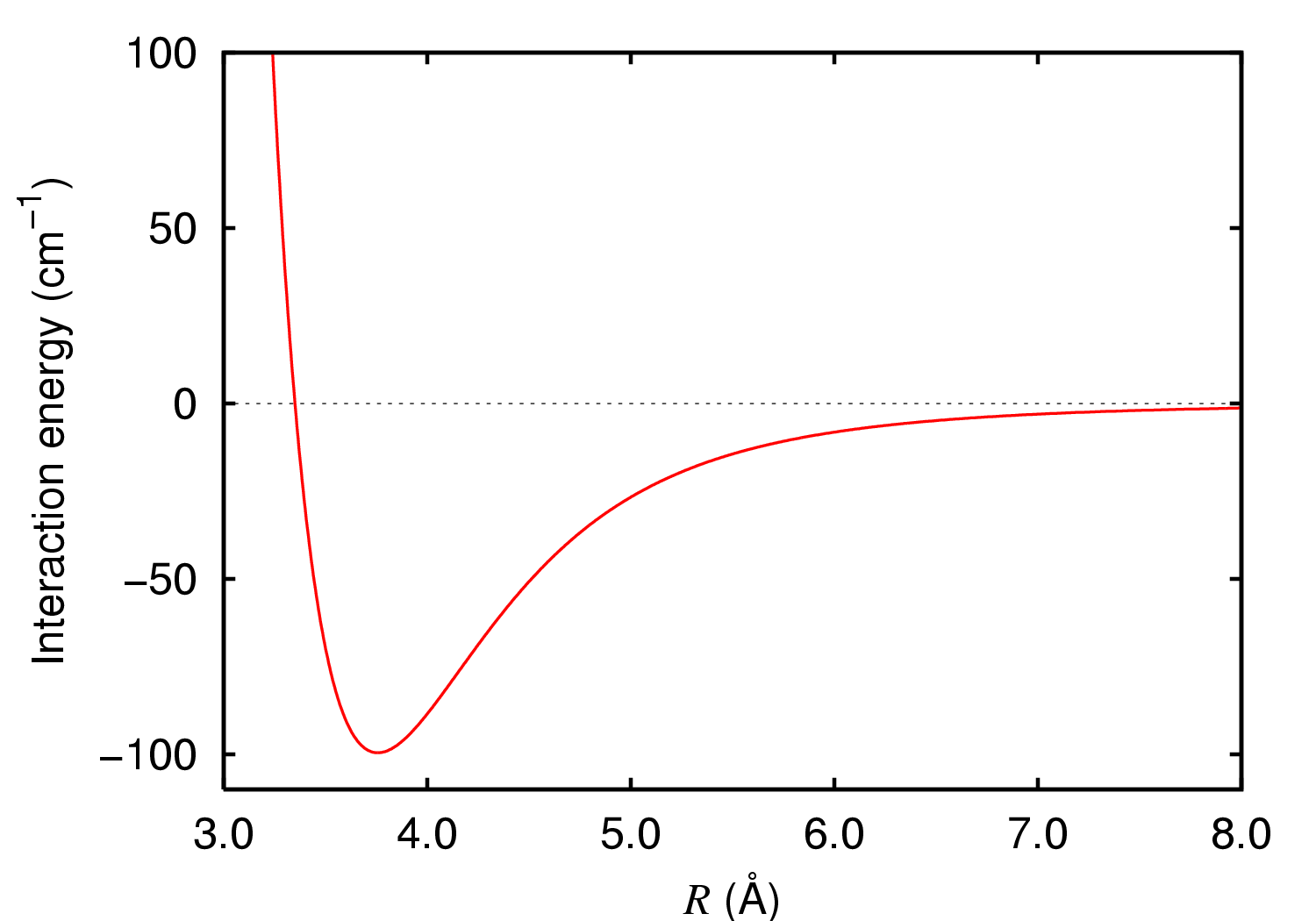
Dispersion Forces
London dispersion forces (LDF, also known as dispersion forces, London forces, instantaneous dipole–induced dipole forces, fluctuating induced dipole bonds or loosely as van der Waals forces) are a type of intermolecular force acting between atoms and molecules that are normally electrically symmetric; that is, the electrons are symmetrically distributed with respect to the nucleus. They are part of the van der Waals forces. The LDF is named after the German physicist Fritz London. They are the weakest intermolecular force. Introduction The electron distribution around an atom or molecule undergoes fluctuations in time. These fluctuations create instantaneous electric fields which are felt by other nearby atoms and molecules, which in turn adjust the spatial distribution of their own electrons. The net effect is that the fluctuations in electron positions in one atom induce a corresponding redistribution of electrons in other atoms, such that the electron motions become corr ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Hamaker Constant
The Hamaker constant ''A'' can be defined for a van der Waals (vdW) body–body interaction: :A=\pi^2C\rho_1\rho_2, where \rho_1 and \rho_2 are the number densities of the two interacting kinds of particles, and ''C'' is the London coefficient in the particle–particle pair interaction. It is named after H. C. Hamaker. The magnitude of this constant reflects the strength of the vdW-force between two particles, or between a particle and a substrate. The Hamaker constant provides the means to determine the interaction parameter ''C'' from the vdW-pair potential, w(r) = -C/r^6. Hamaker's method and the associated Hamaker constant ignores the influence of an intervening medium between the two particles of interaction. In 1956 Lifshitz developed a description of the vdW energy but with consideration of the dielectric properties of this intervening medium (often a continuous phase). The Van der Waals forces are effective only up to several hundred angstroms. When the interactions ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Capillary Pressure
In fluid statics, capillary pressure () is the pressure between two immiscible fluids in a thin tube (see capillary action), resulting from the interactions of forces between the fluids and solid walls of the tube. Capillary pressure can serve as both an opposing or driving force for fluid transport and is a significant property for research and industrial purposes (namely microfluidic design and oil extraction from porous rock). It is also observed in natural phenomena. Definition Capillary pressure is defined as: :p_c=p_-p_ where: :p_is the capillary pressure :p_ is the pressure of the non-wetting phase :p_ is the pressure of the wetting phase The wetting phase is identified by its ability to preferentially diffuse across the capillary walls before the non-wetting phase. The "wettability" of a fluid depends on its surface tension, the forces that drive a fluid's tendency to take up the minimal amount of space possible, and it is determined by the contact angle of the fluid.Fan ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Capillary Condensation
Capillary condensation is the "process by which multilayer adsorption from the vapor haseinto a porous medium proceeds to the point at which pore spaces become filled with condensed liquid from the vapor hase"Schramm, L.L ''The Language of Colloid & Interface Science'' 1993, ACS Professional Reference Book, ACS: Washington, D.C. The unique aspect of capillary condensation is that vapor condensation occurs below the saturation vapor pressure, Psat, of the pure liquid.Hunter, R.J. ''Foundations of Colloid Science'' 2nd Edition, Oxford University Press, 2001. This result is due to an increased number of van der Waals interactions between vapor phase molecules inside the confined space of a capillary. Once condensation has occurred, a meniscus immediately forms at the liquid-vapor interface which allows for equilibrium below the saturation vapor pressure. Meniscus formation is dependent on the surface tension of the liquid and the shape of the capillary, as shown by the Young-Lap ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Surface Tension
Surface tension is the tendency of liquid surfaces at rest to shrink into the minimum surface area possible. Surface tension is what allows objects with a higher density than water such as razor blades and insects (e.g. water striders) to float on a water surface without becoming even partly submerged. At liquid–air interfaces, surface tension results from the greater attraction of liquid molecules to each other (due to cohesion) than to the molecules in the air (due to adhesion). There are two primary mechanisms in play. One is an inward force on the surface molecules causing the liquid to contract. Second is a tangential force parallel to the surface of the liquid. This ''tangential'' force is generally referred to as the surface tension. The net effect is the liquid behaves as if its surface were covered with a stretched elastic membrane. But this analogy must not be taken too far as the tension in an elastic membrane is dependent on the amount of deformation of th ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Contact Angle
The contact angle is the angle, conventionally measured through the liquid, where a liquid–vapor interface meets a solid surface. It quantifies the wettability of a solid surface by a liquid via the Young equation. A given system of solid, liquid, and vapor at a given temperature and pressure has a unique equilibrium contact angle. However, in practice a dynamic phenomenon of contact angle hysteresis is often observed, ranging from the advancing (maximal) contact angle to the receding (minimal) contact angle. The equilibrium contact is within those values, and can be calculated from them. The equilibrium contact angle reflects the relative strength of the liquid, solid, and vapour molecular interaction. The contact angle depends upon the medium above the free surface of the liquid, and the nature of the liquid and solid in contact. It is independent of the inclination of solid to the liquid surface. It changes with surface tension and hence with the temperature and purity of ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Meniscus (liquid)
The meniscus (plural: ''menisci'', from the Greek for "crescent") is the curve in the upper surface of a liquid close to the surface of the container or another object, produced by surface tension. A concave meniscus occurs when the attraction between the particles of the liquid and the container ( adhesion) is more than half the attraction of the particles of the liquid to each other ( cohesion), causing the liquid to climb the walls of the container (see surface tension#Causes). This occurs between water and glass. Water-based fluids like sap, honey, and milk also have a concave meniscus in glass or other wettable containers. Conversely, a convex meniscus occurs when the adhesion energy is less than half the cohesion energy. Convex menisci occur, for example, between mercury and glass in barometers and thermometers. In general, the shape of the surface of a liquid can be complex. For a sufficiently small circular tube, the shape of the meniscus will approximate a sect ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Adsorption
Adsorption is the adhesion of atoms, ions or molecules from a gas, liquid or dissolved solid to a surface. This process creates a film of the ''adsorbate'' on the surface of the ''adsorbent''. This process differs from absorption, in which a fluid (the ''absorbate'') is dissolved by or permeates a liquid or solid (the ''absorbent''). Adsorption is a ''surface phenomenon'', while absorption involves the whole volume of the material, although adsorption does often precede absorption. The term ''sorption'' encompasses both processes, while '' desorption'' is the reverse of it. Like surface tension, adsorption is a consequence of surface energy. In a bulk material, all the bonding requirements (be they ionic, covalent or metallic) of the constituent atoms of the material are fulfilled by other atoms in the material. However, atoms on the surface of the adsorbent are not wholly surrounded by other adsorbent atoms and therefore can attract adsorbates. The exact nature of the b ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Disjoining Pressure
In surface chemistry, disjoining pressure (symbol ) according to an IUPAC definition arises from an attractive interaction between two surfaces. For two flat and parallel surfaces, the value of the disjoining pressure (i.e., the force per unit area) can be calculated as the derivative of the Gibbs energy of interaction per unit area in respect to distance (in the direction normal to that of the interacting surfaces). There is also a related concept of disjoining force, which can be viewed as disjoining pressure times the surface area of the interacting surfaces. The concept of disjoining pressure was introduced by Derjaguin (1936) as the difference between the pressure in a region of a phase adjacent to a surface confining it, and the pressure in the bulk of this phase. Description Disjoining pressure can be expressed as:Hans-Jürgen Butt, Karlheinz Graf, Michael Kappl,"Physics and chemistry of interfaces", John Wiley & Sons Canada, Ltd., 1 edition, 2003, page 9(Google book ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
IUPAC
The International Union of Pure and Applied Chemistry (IUPAC ) is an international federation of National Adhering Organizations working for the advancement of the chemical sciences, especially by developing nomenclature and terminology. It is a member of the International Science Council (ISC). IUPAC is registered in Zürich, Switzerland, and the administrative office, known as the "IUPAC Secretariat", is in Research Triangle Park, North Carolina, United States. This administrative office is headed by IUPAC's executive director, currently Lynn Soby. IUPAC was established in 1919 as the successor of the International Congress of Applied Chemistry for the advancement of chemistry. Its members, the National Adhering Organizations, can be national chemistry societies, national academies of sciences, or other bodies representing chemists. There are fifty-four National Adhering Organizations and three Associate National Adhering Organizations. IUPAC's Inter-divisional Committee o ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
SI Units
The International System of Units, known by the international abbreviation SI in all languages and sometimes pleonastically as the SI system, is the modern form of the metric system and the world's most widely used system of measurement. Established and maintained by the General Conference on Weights and Measures (CGPM), it is the only system of measurement with an official status in nearly every country in the world, employed in science, technology, industry, and everyday commerce. The SI comprises a coherent system of units of measurement starting with seven base units, which are the second (symbol s, the unit of time), metre (m, length), kilogram (kg, mass), ampere (A, electric current), kelvin (K, thermodynamic temperature), mole (mol, amount of substance), and candela (cd, luminous intensity). The system can accommodate coherent units for an unlimited number of additional quantities. These are called coherent derived units, which can always be represented as pro ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |