|
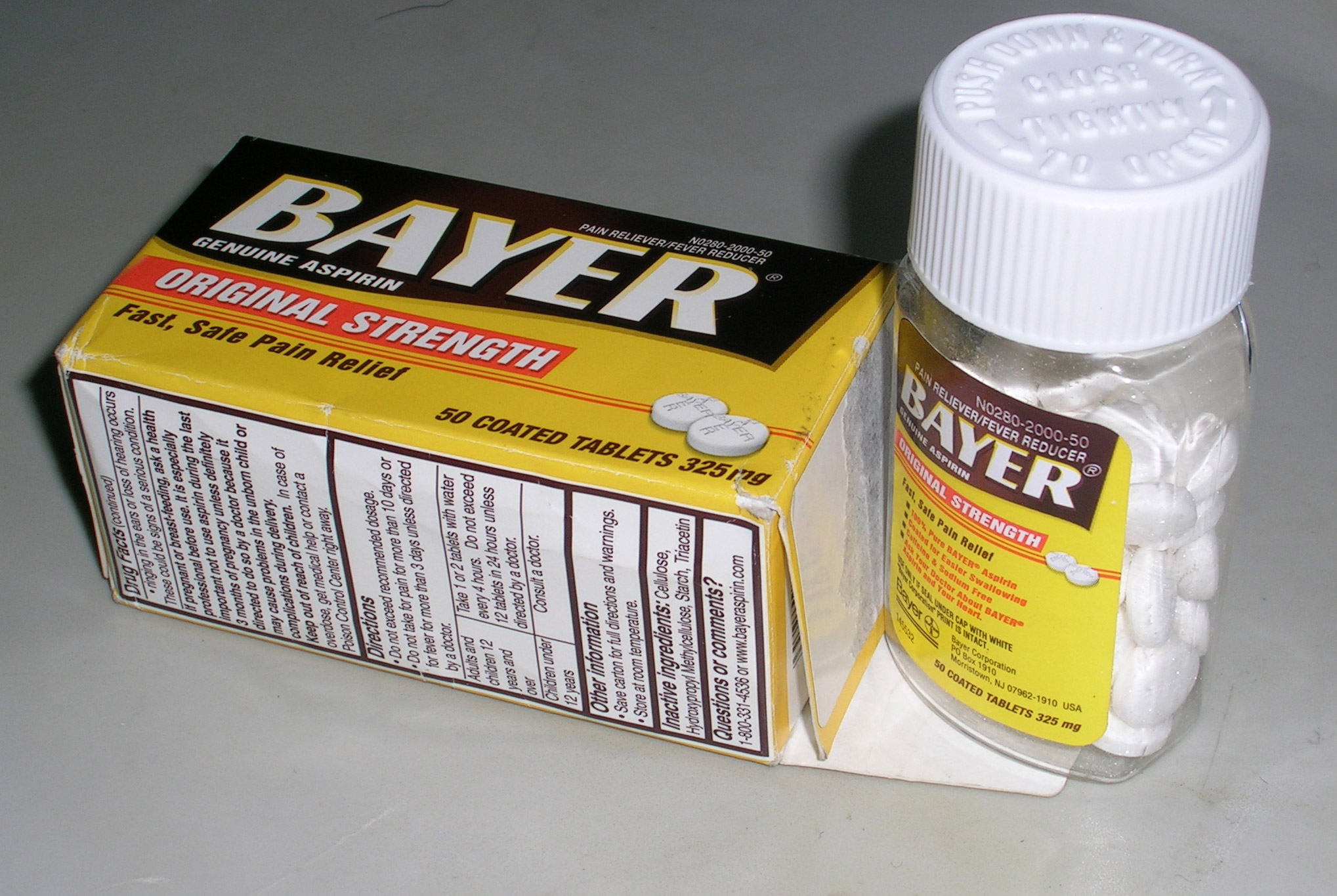
Drug Identification Number
Any product defined as a drug under the Canadian Food and Drugs Act must have an associated drug identification number (or DIN). A DIN also pertains to veterinary drugs permitted for sale in Canada. The drug identification number (DIN) is the 8 digit number located on the label of prescription and over-the-counter drug products that have been evaluated by the Therapeutic Products Directorate (TPD) and approved for sale in Canada. Once a drug has been approved, the Therapeutic Products Directorate issues a DIN, which permits the manufacturer to market the drug in Canada. For drugs, where there is minimal market history in Canada, there is a more stringent review and the drug is required to have a Notice of Compliance and a DIN in order to be marketed in Canada. A DIN lets the user know that the product has undergone and passed a review of its formulation, labeling, and instructions for use. A drug product sold in Canada without a DIN is not in compliance with Canadian law, with li ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Drug
A drug is any chemical substance that causes a change in an organism's physiology or psychology when consumed. Drugs are typically distinguished from food and substances that provide nutritional support. Consumption of drugs can be via insufflation (medicine), inhalation, drug injection, injection, smoking, ingestion, absorption (skin), absorption via a dermal patch, patch on the skin, suppository, or sublingual administration, dissolution under the tongue. In pharmacology, a drug is a chemical substance, typically of known structure, which, when administered to a living organism, produces a biological effect. A pharmaceutical drug, also called a medication or medicine, is a chemical substance used to pharmacotherapy, treat, cure, preventive healthcare, prevent, or medical diagnosis, diagnose a disease or to promote well-being. Traditionally drugs were obtained through extraction from medicinal plants, but more recently also by organic synthesis. Pharmaceutical drugs may be used ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Canada
Canada is a country in North America. Its ten provinces and three territories extend from the Atlantic Ocean to the Pacific Ocean and northward into the Arctic Ocean, covering over , making it the world's second-largest country by total area. Its southern and western border with the United States, stretching , is the world's longest binational land border. Canada's capital is Ottawa, and its three largest metropolitan areas are Toronto, Montreal, and Vancouver. Indigenous peoples have continuously inhabited what is now Canada for thousands of years. Beginning in the 16th century, British and French expeditions explored and later settled along the Atlantic coast. As a consequence of various armed conflicts, France ceded nearly all of its colonies in North America in 1763. In 1867, with the union of three British North American colonies through Confederation, Canada was formed as a federal dominion of four provinces. This began an accretion of provinces an ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Food And Drugs Act
The ''Food and Drugs Act'' (the ''Act'') (formal title ''An Act respecting food, drugs, cosmetics and therapeutic devices'') is an act of the Parliament of Canada regarding the production, import, export, transport across provinces and sale of food, drugs, contraceptive devices and cosmetics (including personal cleaning products such as soap and toothpaste). It was first passed in 1920 and most recently revised in 1985. It attempts to ensure that these products are safe, that their ingredients are disclosed and that drugs are effective and are not sold as food or cosmetics. It also states that cures for disease listed in Schedule A (including cancer, obesity, anxiety, asthma, depression, appendicitis, and sexually transmitted diseases), cannot be advertised to the general public. Background After the launch of the Federal Department of Health in 1919, the ''Food and Drugs Act'' was presented in late 1920. Rules and regulations developed under the ''Act'' established the require ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Over-the-counter Drug
Over-the-counter (OTC) drugs are medicines sold directly to a consumer without a requirement for a prescription from a healthcare professional, as opposed to prescription drugs A prescription drug (also prescription medication or prescription medicine) is a pharmaceutical drug that legally requires a medical prescription to be dispensed. In contrast, over-the-counter drugs can be obtained without a prescription. The rea ..., which may be supplied only to consumers possessing a valid prescription. In many countries, OTC drugs are selected by a regulatory agency to ensure that they contain ingredients that are safe and effective when used without a physician's care. OTC drugs are usually regulated according to their active pharmaceutical ingredient (API) rather than final products. By regulating APIs instead of specific drug formulations, governments allow manufacturers the freedom to formulate ingredients, or combinations of ingredients, into proprietary mixtures. The term ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Therapeutic Products Directorate
Pharmaceutical Drugs Directorate (PDD) is a Canadian federal authority that regulates small molecule pharmaceutical drugs for human use. Prior to being given market authorization, a manufacturer must present substantive scientific evidence of a product's safety, efficacy, and quality as required by the Food and Drugs Act and Regulations. It is one of the ten operational directorates of the Health Products and Food Branch, a branch of Health Canada. See also *European Medicines Agency *Good Manufacturing Practice *Good clinical practice *Informed consent *Institutional review board An institutional review board (IRB), also known as an independent ethics committee (IEC), ethical review board (ERB), or research ethics board (REB), is a committee that applies research ethics by reviewing the methods proposed for research to ens ... * International Conference on Harmonisation of Technical Requirements for Registration of Pharmaceuticals for Human Use * Investigational product * Invest ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Pharmaceutical Code
Pharmaceutical codes are used in medical classification to uniquely identify medication. They may uniquely identify an active ingredient, drug system (including inactive ingredients and time-release agents) in general, or a specific pharmaceutical product from a specific manufacturer. Examples Drug system identifiers (manufacturer-specific including inactive ingredients): * National Drug Code (NDC) — administered by Food and Drug Administration. * Drug Identification Number (DIN) — administered by Health Canada under the Food and Drugs Act * Hong Kong Drug Registration — administered by the Pharmaceutical Service of the Department of Health (Hong Kong) * National Pharmaceutical Product Index - South Africa Hierarchical systems: * Anatomical Therapeutic Chemical Classification System (AT, or ATC/DDD) — administered by World Health Organization * Generic Product Identifier (GPI) — hierarchical classification number published by MediSpan * SNOMED — C axis Ingredients: ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |