|
Difluoroamino Sulfur Pentafluoride
Difluoroamino sulfur pentafluoride is a gaseous chemical compound of fluorine, sulfur, and nitrogen. It is unusual in having a hexa-coordinated sulfur atom with a link to nitrogen. Other names for this substance include difluoro(pentafluorosulfur)amine, pentafluorosulfanyldifluoramine, and pentafluorosulfanyl ''N'',''N''-difluoramine. Properties Difluoroamino sulfur pentafluoride is a colourless gas at room temperature. The molecule is shaped as a tetragonal bipyramid around the sulfur atom. To within half a degree the boiling point is -17.5 °C. Difluoroamino sulfur pentafluoride is stable at room temperature, but decomposes on the timescale of hours at 80 °C. Decomposition results in sulfur tetrafluoride and nitrogen trifluoride. It is not stable above 220 °C. It is stable with water or stainless steel. When stored in quartz and exposed to ultraviolet light, it decomposes slightly and reacts with silica to make , , , , , and . The bond between sulfur and ni ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Sulfur Tetrafluoride
Sulfur tetrafluoride is the chemical compound with the formula S F4. It is a colorless corrosive gas that releases dangerous HF upon exposure to water or moisture. Despite these unwelcome characteristics, this compound is a useful reagent for the preparation of organofluorine compounds, some of which are important in the pharmaceutical and specialty chemical industries. Structure Sulfur in SF4 is in the formal +4 oxidation state. Of sulfur's total of six valence electrons, two form a lone pair. The structure of SF4 can therefore be anticipated using the principles of VSEPR theory: it is a see-saw shape, with S at the center. One of the three equatorial positions is occupied by a nonbonding lone pair of electrons. Consequently, the molecule has two distinct types of F ligands, two axial and two equatorial. The relevant bond distances are = 164.3 pm and = 154.2 pm. It is typical for the axial ligands in hypervalent molecules to be bonded less strongly. In c ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
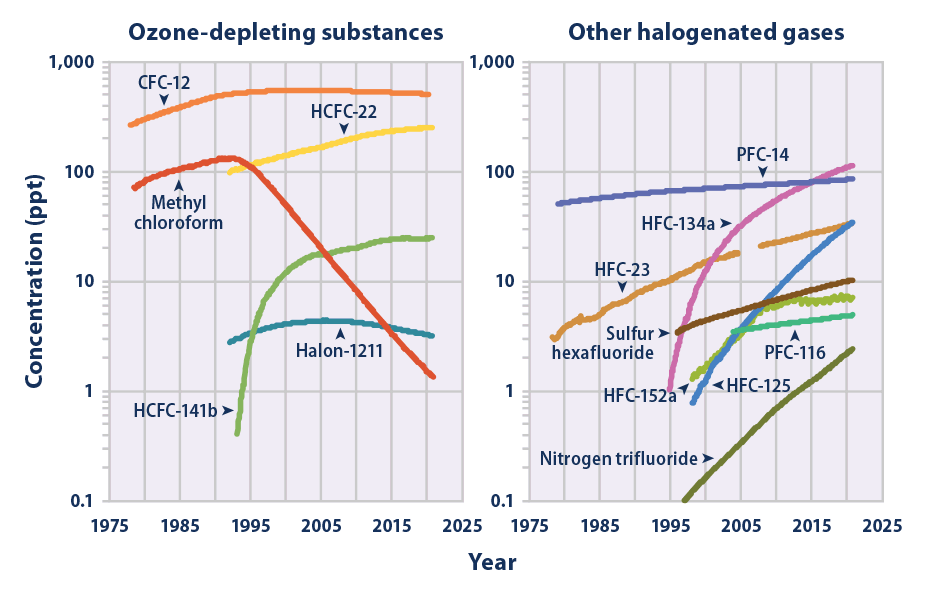
Nitrogen Trifluoride
Nitrogen trifluoride () is an inorganic, colorless, non-flammable, toxic gas with a slightly musty odor. It finds increasing use within the manufacturing of flat-panel displays, photovoltaics, LEDs and other microelectronics. Nitrogen trifluoride is also an extremely strong and long-lived greenhouse gas. Its atmospheric burden exceeded 2 parts per trillion during 2019 and has doubled every five years since the late 20th century. Synthesis and reactivity Nitrogen trifluoride did not exist in significant quantities on Earth prior to its synthesis by humans. It is a rare example of a binary fluoride that can be prepared directly from the elements only at very uncommon conditions, such as an electric discharge. After first attempting the synthesis in 1903, Otto Ruff prepared nitrogen trifluoride by the electrolysis of a molten mixture of ammonium fluoride and hydrogen fluoride. It proved to be far less reactive than the other nitrogen trihalides nitrogen trichloride, nitrogen trib ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Disulfur Decafluoride
Disulfur decafluoride is a chemical compound with the formula . It was discovered in 1934 by Kenneth Denbigh, Denbigh and Whytlaw-Gray. Each sulfur atom of the molecule is octahedral, and surrounded by five fluorine atoms and one sulfur atom. The two sulfur atoms are connected by a single bond. In the molecule, the oxidation state of each sulfur atoms is +5, but their Valence (chemistry), valency is 6 (they are hexavalent). is highly toxic, with toxicity four times that of phosgene. It is a colorless liquid with a burnt match smell similar to sulfur dioxide. Production Disulfur decafluoride is produced by photolysis of : : Disulfur decafluoride arises by the decomposition of sulfur hexafluoride. It is produced by the electrical decomposition of sulfur hexafluoride ()—an essentially inert Electrical insulation, insulator used in high voltage systems such as transmission lines, Electrical substation, substations and switchgear. is also made during the production of . Propert ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Dinitrogen Tetrafluoride
Tetrafluorohydrazine or perfluorohydrazine, , is a colourless, reactive inorganic gas. It is a fluorinated analog of hydrazine. It is a highly hazardous chemical that explodes in the presence of organic materials. Tetrafluorohydrazine is manufactured from nitrogen trifluoride using an iron catalyst or iron(II) fluoride. It is used in some chemical syntheses, as a precursor or a catalyst. Tetrafluorohydrazine was considered for use as a high-energy liquid oxidizer in some never-flown rocket fuel formulas in 1959. Properties Tetrafluorohydrazine is in equilibrium with its radical monomer nitrogen difluoride. : N2F4 2 NF2• At room temperature N2F4 is mostly associated with only 0.7% in the form of NF2 at 5mm Hg pressure. When the temperature rises to 225 °C, it mostly dissociates with 99% in the form of NF2. molecule dimensions and angles The energy needed to break the N-N bond in N2F4 is 20.8 kcal/mol, with an entropy change of 38.6 eu. For comparison, the dis ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Sulfur Chloride Pentafluoride
Sulfur chloride pentafluoride is an inorganic compound with the formula . It exists as a colorless gas at room temperature and is highly toxic, like most inorganic compounds containing the pentafluorosulfide (–) functional group.Nyman, F., Roberts, H. L., Seaton, T. "Sulfur Chloride Pentafluoride" Inorganic Syntheses, 1966, Volume 8, p. 160. The compound adopts an octahedral geometry with symmetry. Sulfur chloride pentafluoride is the only commercially available reagent for adding the – group to organic compounds. Reactivity is highly reactive and toxic. In contrast, sulfur hexafluoride () is inert and nontoxic despite having a closely related chemical formula. This difference highlights the lability of the S–Cl bond in . Under free-radical conditions, adds across double bonds. The following reaction occurs with propene: : + → CH3CHClCH2SF5 The addition reaction is catalyzed by at around −30 °C. is used similarly. is also a precursor to O(SF5)2 and F2NSF ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Nitrogen Difluoride
Nitrogen difluoride, also known as difluoroamino, is a reactive radical molecule with formula . This small molecule is in equilibrium with its dimer dinitrogen tetrafluoride. : As the temperature increases the proportion of increases. The molecule is unusual in that it has an odd number of electrons, yet is stable enough to study experimentally. Properties The energy needed to break the N–N bond in is , with an entropy change of 38.6 eu. molecule dimensions and angles For comparison, the dissociation energy of the N–N bond is in , in , and in . The enthalpy of formation of (Δ''H''f) is . At room temperature is mostly associated with only 0.7% in the form of at pressure. When the temperature rises to 225 °C, it mostly dissociates with 99% in the form of . In , the N–F bond length is 1.3494 Å and the angle subtended at F–N–F is 103.33°. In the infrared spectrum the N–F bond in has a symmetrical stretching frequency of 1075 cm� ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Sulfur Dioxide
Sulfur dioxide (IUPAC-recommended spelling) or sulphur dioxide (traditional Commonwealth English) is the chemical compound with the formula . It is a toxic gas responsible for the odor of burnt matches. It is released naturally by volcanic activity and is produced as a by-product of copper extraction and the burning of sulfur- bearing fossil fuels. Structure and bonding SO2 is a bent molecule with ''C''2v symmetry point group. A valence bond theory approach considering just ''s'' and ''p'' orbitals would describe the bonding in terms of resonance between two resonance structures. The sulfur–oxygen bond has a bond order of 1.5. There is support for this simple approach that does not invoke ''d'' orbital participation. In terms of electron-counting formalism, the sulfur atom has an oxidation state of +4 and a formal charge of +1. Occurrence Sulfur dioxide is found on Earth and exists in very small concentrations and in the atmosphere at about 1 ppm. On other planets, ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Thiophosgene
Thiophosgene is a red liquid with the formula . It is a molecule with trigonal planar geometry. There are two reactive C–Cl bonds that allow it to be used in diverse organic syntheses. Preparation is prepared in a two-step process from carbon disulfide. In the first step, carbon disulfide is chlorinated to give trichloromethanesulfenyl chloride (perchloromethyl mercaptan), : : The chlorination must be controlled as excess chlorine converts trichloromethanesulfenyl chloride into carbon tetrachloride Carbon tetrachloride, also known by many other names (such as tetrachloromethane, also IUPAC nomenclature of inorganic chemistry, recognised by the IUPAC, carbon tet in the cleaning industry, Halon-104 in firefighting, and Refrigerant-10 in HVAC .... Steam distillation separates the trichloromethanesulfenyl chloride, a rare sulfenyl chloride, and hydrolyzes the sulfur monochloride. Reduction of trichloromethanesulfenyl chloride produces thiophosgene: : Tin and dihydroanthracene ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Sulfur Hexafluoride
Sulfur hexafluoride or sulphur hexafluoride (British spelling) is an inorganic compound with the formula SF6. It is a colorless, odorless, non- flammable, and non-toxic gas. has an octahedral geometry, consisting of six fluorine atoms attached to a central sulfur atom. It is a hypervalent molecule. Typical for a nonpolar gas, is poorly soluble in water but quite soluble in nonpolar organic solvents. It has a density of 6.12 g/L at sea level conditions, considerably higher than the density of air (1.225 g/L). It is generally transported as a liquefied compressed gas. is 23,500 times more potent than as a greenhouse gas but exists in relatively minor concentrations in the atmosphere. Its concentration in Earth's troposphere reached 10.63 parts per trillion (ppt) in 2021, rising at 0.39 ppt/year. The increase over the prior 40 years was driven in large part by the expanding electric power sector, including fugitive emissions from banks of gas contained in its med ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Nitrogen
Nitrogen is the chemical element with the symbol N and atomic number 7. Nitrogen is a nonmetal and the lightest member of group 15 of the periodic table, often called the pnictogens. It is a common element in the universe, estimated at seventh in total abundance in the Milky Way and the Solar System. At standard temperature and pressure, two atoms of the element bond to form N2, a colorless and odorless diatomic gas. N2 forms about 78% of Earth's atmosphere, making it the most abundant uncombined element. Nitrogen occurs in all organisms, primarily in amino acids (and thus proteins), in the nucleic acids ( DNA and RNA) and in the energy transfer molecule adenosine triphosphate. The human body contains about 3% nitrogen by mass, the fourth most abundant element in the body after oxygen, carbon, and hydrogen. The nitrogen cycle describes the movement of the element from the air, into the biosphere and organic compounds, then back into the atmosphere. Many indus ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Alkene
In organic chemistry, an alkene is a hydrocarbon containing a carbon–carbon double bond. Alkene is often used as synonym of olefin, that is, any hydrocarbon containing one or more double bonds.H. Stephen Stoker (2015): General, Organic, and Biological Chemistry'. 1232 pages. Two general types of monoalkenes are distinguished: terminal and internal. Also called α-olefins, terminal alkenes are more useful. However, the International Union of Pure and Applied Chemistry (IUPAC) recommends using the name "alkene" only for acyclic hydrocarbons with just one double bond; alkadiene, alkatriene, etc., or polyene for acyclic hydrocarbons with two or more double bonds; cycloalkene, cycloalkadiene, etc. for cyclic ones; and "olefin" for the general class – cyclic or acyclic, with one or more double bonds. Acyclic alkenes, with only one double bond and no other functional groups (also known as mono-enes) form a homologous series of hydrocarbons with the general formula with '' ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Sulfur Fluorides
Sulfur (or sulphur in British English) is a chemical element with the symbol S and atomic number 16. It is abundant, multivalent and nonmetallic. Under normal conditions, sulfur atoms form cyclic octatomic molecules with a chemical formula S8. Elemental sulfur is a bright yellow, crystal A crystal or crystalline solid is a solid material whose constituents (such as atoms, molecules, or ions) are arranged in a highly ordered microscopic structure, forming a crystal lattice that extends in all directions. In addition, macros ...line solid at room temperature. Sulfur is the tenth most abundant element by mass in the universe and the fifth most on Earth. Though sometimes found in pure, native element minerals, native form, sulfur on Earth usually occurs as sulfide minerals, sulfide and sulfate minerals. Being abundant in native form, sulfur was known in ancient times, being mentioned for its uses in ancient India, ancient Greece, history of China#Ancient China, China, ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |