|
Diethyldithiocarbamate
Sodium diethyldithiocarbamate is the organosulfur compound with the formula NaS2CN(C2H5)2. It is a pale yellow, water soluble salt. Preparation This salt is obtained by treating carbon disulfide with diethylamine in the presence of sodium hydroxide: :CS2 + HN(C2H5)2 + NaOH → NaS2CN(C2H5)2 + H2O Other dithiocarbamates can be prepared similarly from secondary amines and carbon disulfide. They are used as chelating agents for transition metal ions and as precursors to herbicides and vulcanization reagents. Oxidation to thiuram disulfide Oxidation of sodium diethyldithiocarbamate gives the disulfide, also called a thiuram disulfide (Et = ethyl): :2 NaS2CNEt2 + I2 → Et2NC(S)S-SC(S)NEt2 + 2 NaI Ligand bonding The diethyldithiocarbamate anion forms the basis of transition metal dithiocarbamate complexes. The ligands coordinate to many " softer" metals via the two sulfur atoms. Other more complicated bonding modes are known including binding as unidentate liga ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Transition Metal Dithiocarbamate Complexes
133px, Tris(dithiocarbamate) complexes have idealized D3 symmetry. Transition metal dithiocarbamate complexes are coordination complexes containing one or more dithiocarbamate ligand, which are typically abbreviated R2dtc−. Many complexes are known. Several homoleptic derivatives have the formula M(R2dtc)n where n = 2 and 3. Ligand characteristics Dithiocarbamates are anions. Because of the pi-donor properties of the amino substituent, the two sulfur centers show enhanced basicity. This situation is represented by the zwitterionic resonance structure that depicts a positive charge on N and negative charges on both sulfurs. This N to C pi-bonding results in partial double bond character. Consequently, barriers to rotational about this bond are elevated. Another consequence of their high basicity, dithiocarbamates often stabilize complexes in uncharacteristically high oxidation state (e.g., Fe(IV), Co(IV), Ni(III), Cu(III)). Dithiocarbamate salts are easily synthesized. ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Carbon Disulfide
Carbon disulfide (also spelled as carbon disulphide) is a neurotoxic, colorless, volatile liquid with the formula and structure . The compound is used frequently as a building block in organic chemistry as well as an industrial and chemical non-polar solvent. It has an "ether-like" odor, but commercial samples are typically contaminated with foul-smelling impurities.. It is of comparable toxicity to carbon monoxide. History In 1796, the German chemist Wilhelm August Lampadius (1772–1842) first prepared carbon disulfide by heating pyrite with moist charcoal. He called it "liquid sulfur" (''flüssig Schwefel''). The composition of carbon disulfide was finally determined in 1813 by the team of the Swedish chemist Jöns Jacob Berzelius (1779–1848) and the Swiss-British chemist Alexander Marcet (1770–1822). Their analysis was consistent with an empirical formula of CS2. Occurrence, manufacture, properties Small amounts of carbon disulfide are released by volcanic eruptio ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Thiuram Disulfide
Thiuram disulfides are a class of organosulfur compounds with the formula (R2NCSS)2. Many examples are known, but popular ones include R = Me and Et. They are disulfides obtained by oxidation of the dithiocarbamates. These compounds are used in sulfur vulcanization of rubber as well as pesticides and drugs. They are typically white or pale yellow solids that are soluble in organic solvents. Preparation, structure, reactions They are prepared by the oxidation of the salts of the corresponding dithiocarbamates (e.g. sodium diethyldithiocarbamate). Typical oxidants are chlorine and hydrogen peroxide: :2 R2NCSSNa + Cl2 → (R2NCSS)2 + 2 NaCl Thiuram disulfides react with Grignard reagents to give esters of dithiocarbamic acid, as in the preparation of methyl dimethyldithiocarbamate: : e2NC(S)Ssub>2 + MeMgX → Me2NC(S)SMe + Me2NCS2MgX The compounds feature planar dithiocarbamate subunits and are linked by an S−S bond of 2.00 Å. The C(S)−N bond is sh ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Dithiocarbamate
In organic chemistry, a dithiocarbamate is a functional group with the general formula and structure . It is the analog of a carbamate in which both oxygen atoms are replaced by sulfur atoms (when only 1 oxygen is replaced the result is thiocarbamate). A common example is sodium diethyldithiocarbamate. Dithiocarbamates and their derivatives are widely used in the vulcanization of rubber. Formation Many primary and secondary amines react with carbon disulfide and sodium hydroxide to form dithiocarbamate salts: :R2NH + CS2 + NaOH → R2NCS2−Na+ + H2O Ammonia reacts with CS2 similarly: :2 NH3 + CS2 → H2NCS2−NH4+ Dithiocarbamate salts are pale colored solids that are soluble in water and polar organic solvents. Reactions Dithiocarbamates are readily S-alkylated. Thus, methyl dimethyldithiocarbamate can be prepared by methylation of the dithiocarbamate: :(CH3)2NCS2Na + (CH3O)2SO2 → (CH3)2NC(S)SCH3 + Na H3OSO3 Oxidation of dithiocarbamates gives th ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Superoxide Dismutase
Superoxide dismutase (SOD, ) is an enzyme that alternately catalyzes the dismutation (or partitioning) of the superoxide () radical into ordinary molecular oxygen (O2) and hydrogen peroxide (). Superoxide is produced as a by-product of oxygen metabolism and, if not regulated, causes many types of cell damage. Hydrogen peroxide is also damaging and is degraded by other enzymes such as catalase. Thus, SOD is an important antioxidant defense in nearly all living cells exposed to oxygen. One exception is ''Lactobacillus plantarum'' and related lactobacilli, which use a different mechanism to prevent damage from reactive . Chemical reaction SODs catalyze the disproportionation of superoxide: : 2 HO2 → O2 + H2O2 In this way, is converted into two less damaging species. The pathway by which SOD-catalyzed dismutation of superoxide may be written, for Cu,Zn SOD, with the following reactions: * Cu2+-SOD + → Cu+-SOD + O2 (reduction of copper; oxidation of superoxide) * Cu+-S ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Antioxidant
Antioxidants are compounds that inhibit oxidation, a chemical reaction that can produce free radicals. This can lead to polymerization and other chain reactions. They are frequently added to industrial products, such as fuels and lubricants, to prevent oxidation, and to foods to prevent spoilage, in particular the rancidification of oils and fats. In cells, antioxidants such as glutathione, mycothiol or bacillithiol, and enzyme systems like superoxide dismutase, can prevent damage from oxidative stress. The only dietary antioxidants are vitamins A, C, and E, but the term ''antioxidant'' has also been applied to numerous other dietary compounds that only have antioxidant properties in vitro, with little evidence for antioxidant properties in vivo. Dietary supplements marketed as antioxidants have not been shown to maintain health or prevent disease in humans. History As part of their adaptation from marine life, terrestrial plants began producing non-marine antioxi ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Angiogenesis
Angiogenesis is the physiological process through which new blood vessels form from pre-existing vessels, formed in the earlier stage of vasculogenesis. Angiogenesis continues the growth of the vasculature by processes of sprouting and splitting. Vasculogenesis is the embryonic formation of endothelial cells from mesoderm cell precursors, and from neovascularization, although discussions are not always precise (especially in older texts). The first vessels in the developing embryo form through vasculogenesis, after which angiogenesis is responsible for most, if not all, blood vessel growth during development and in disease. Angiogenesis is a normal and vital process in growth and development, as well as in wound healing and in the formation of granulation tissue. However, it is also a fundamental step in the transition of tumors from a benign state to a malignant one, leading to the use of angiogenesis inhibitors in the treatment of cancer. The essential role of angiogenesis in ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Metastasis
Metastasis is a pathogenic agent's spread from an initial or primary site to a different or secondary site within the host's body; the term is typically used when referring to metastasis by a cancerous tumor. The newly pathological sites, then, are metastases (mets). It is generally distinguished from cancer invasion, which is the direct extension and penetration by cancer cells into neighboring tissues. Cancer occurs after cells are genetically altered to proliferate rapidly and indefinitely. This uncontrolled proliferation by mitosis produces a primary heterogeneic tumour. The cells which constitute the tumor eventually undergo metaplasia, followed by dysplasia then anaplasia, resulting in a malignant phenotype. This malignancy allows for invasion into the circulation, followed by invasion to a second site for tumorigenesis. Some cancer cells known as circulating tumor cells acquire the ability to penetrate the walls of lymphatic or blood vessels, after which they are abl ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
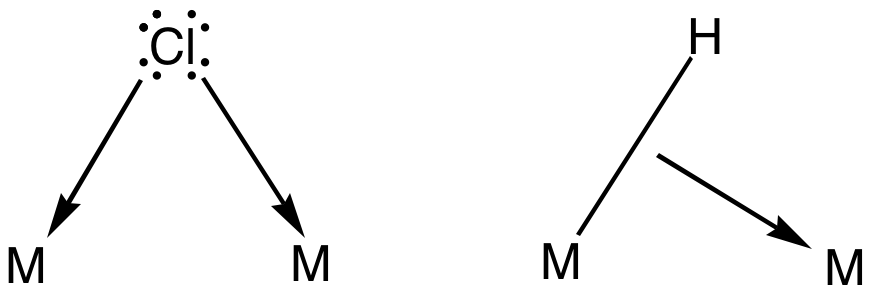
Bridging Ligand
In coordination chemistry, a bridging ligand is a ligand that connects two or more atoms, usually metal ions. The ligand may be atomic or polyatomic. Virtually all complex organic compounds can serve as bridging ligands, so the term is usually restricted to small ligands such as pseudohalides or to ligands that are specifically designed to link two metals. In naming a complex wherein a single atom bridges two metals, the bridging ligand is preceded by the Greek letter mu, μ, with a subscript number denoting the number of metals bound to the bridging ligand. μ2 is often denoted simply as μ. When describing coordination complexes care should be taken not to confuse μ with η ('eta'), which relates to hapticity. Ligands that are not bridging are called terminal ligands. List of bridging ligands Virtually all ligands are known to bridge, with the exception of amines and ammonia. Common bridging ligands include most of the common anions. Many simple organic ligands form str ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Extracellular Matrix
In biology, the extracellular matrix (ECM), also called intercellular matrix, is a three-dimensional network consisting of extracellular macromolecules and minerals, such as collagen, enzymes, glycoproteins and hydroxyapatite that provide structural and biochemical support to surrounding cells. Because multicellularity evolved independently in different multicellular lineages, the composition of ECM varies between multicellular structures; however, cell adhesion, cell-to-cell communication and differentiation are common functions of the ECM. The animal extracellular matrix includes the interstitial matrix and the basement membrane. Interstitial matrix is present between various animal cells (i.e., in the intercellular spaces). Gels of polysaccharides and fibrous proteins fill the Interstitial fluid, interstitial space and act as a compression buffer against the stress placed on the ECM. Basement membranes are sheet-like depositions of ECM on which various epithelial cells rest ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Metalloproteinase
A metalloproteinase, or metalloprotease, is any protease enzyme whose catalytic mechanism involves a metal. An example is ADAM12 which plays a significant role in the fusion of muscle cells during embryo development, in a process known as myogenesis. Most metalloproteases require zinc, but some use cobalt. The metal ion is coordinated to the protein via three ligands. The ligands coordinating the metal ion can vary with histidine, glutamate, aspartate, lysine, and arginine. The fourth coordination position is taken up by a labile water molecule. Treatment with chelating agents such as EDTA leads to complete inactivation. EDTA is a metal chelator that removes zinc, which is essential for activity. They are also inhibited by the chelator orthophenanthroline. Classification There are two subgroups of metalloproteinases: * Exopeptidases, metalloexopeptidases ( EC number: 3.4.17). * Endopeptidases, metalloendopeptidases (3.4.24). Well known metalloendopeptidases include ADAM pr ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Zinc
Zinc is a chemical element with the symbol Zn and atomic number 30. Zinc is a slightly brittle metal at room temperature and has a shiny-greyish appearance when oxidation is removed. It is the first element in group 12 (IIB) of the periodic table. In some respects, zinc is chemically similar to magnesium: both elements exhibit only one normal oxidation state (+2), and the Zn2+ and Mg2+ ions are of similar size.The elements are from different metal groups. See periodic table. Zinc is the 24th most abundant element in Earth's crust and has five stable isotopes. The most common zinc ore is sphalerite (zinc blende), a zinc sulfide mineral. The largest workable lodes are in Australia, Asia, and the United States. Zinc is refined by froth flotation of the ore, roasting, and final extraction using electricity ( electrowinning). Zinc is an essential trace element for humans, animals, plants and for microorganisms and is necessary for prenatal and postnatal development. It ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |