Transition Metal Dithiocarbamate Complexes on:
[Wikipedia]
[Google]
[Amazon]
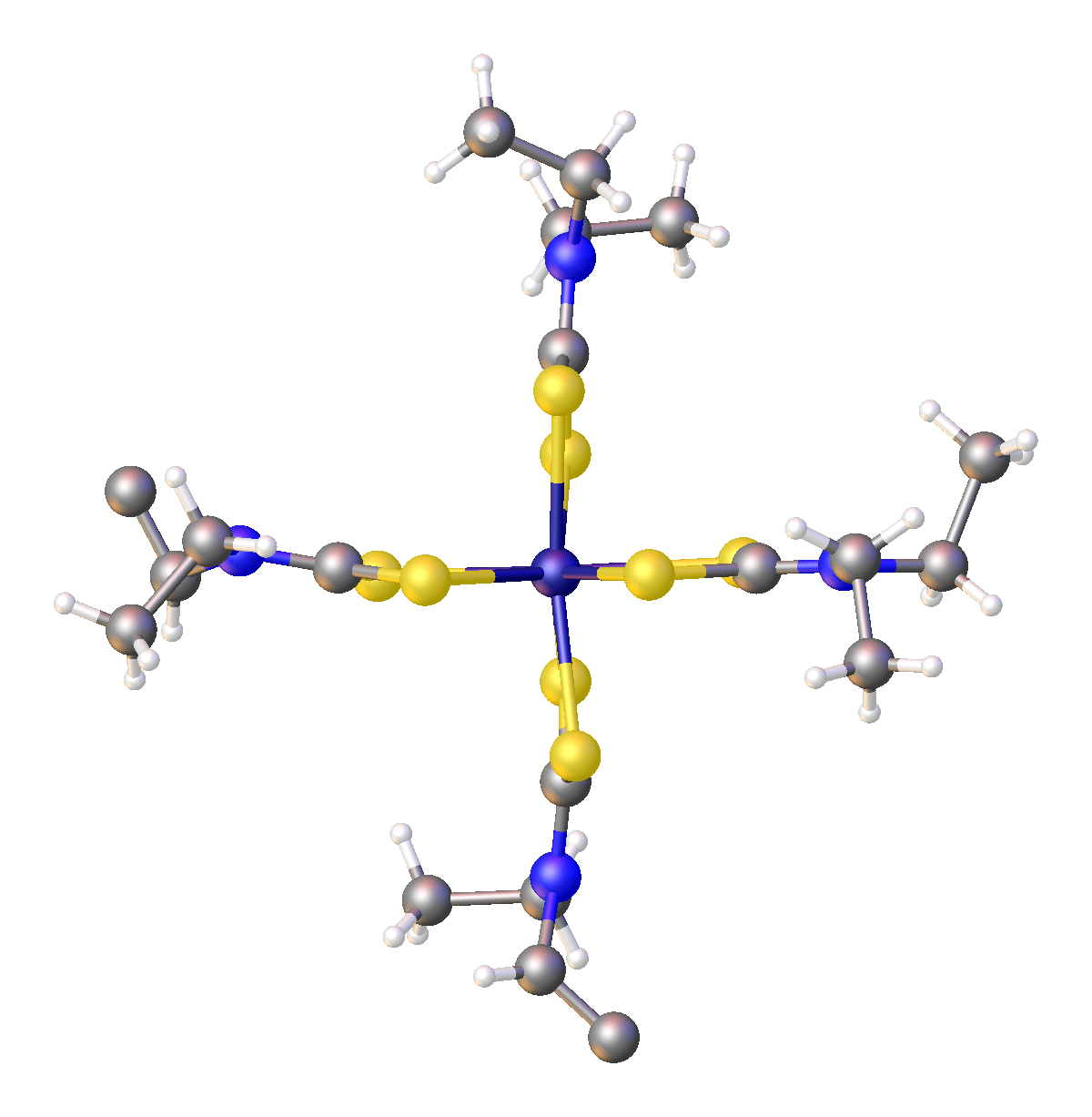
133px, Tris(dithiocarbamate) complexes have idealized D3 symmetry.
Transition metal dithiocarbamate complexes are
 Dithiocarbamates are anions. Because of the pi-donor properties of the amino substituent, the two sulfur centers show enhanced basicity. This situation is represented by the zwitterionic resonance structure that depicts a positive charge on N and negative charges on both sulfurs. This N to C pi-bonding results in partial double bond character. Consequently, barriers to rotational about this bond are elevated. Another consequence of their high basicity, dithiocarbamates often stabilize complexes in uncharacteristically high oxidation state (e.g., Fe(IV), Co(IV), Ni(III), Cu(III)).
Dithiocarbamate salts are easily synthesized. Many primary and secondary
Dithiocarbamates are anions. Because of the pi-donor properties of the amino substituent, the two sulfur centers show enhanced basicity. This situation is represented by the zwitterionic resonance structure that depicts a positive charge on N and negative charges on both sulfurs. This N to C pi-bonding results in partial double bond character. Consequently, barriers to rotational about this bond are elevated. Another consequence of their high basicity, dithiocarbamates often stabilize complexes in uncharacteristically high oxidation state (e.g., Fe(IV), Co(IV), Ni(III), Cu(III)).
Dithiocarbamate salts are easily synthesized. Many primary and secondary
 ;Bis complexes
*
;Bis complexes
* 
coordination complex
A coordination complex consists of a central atom or ion, which is usually metallic and is called the ''coordination centre'', and a surrounding array of bound molecules or ions, that are in turn known as ''ligands'' or complexing agents. Many ...
es containing one or more dithiocarbamate
In organic chemistry, a dithiocarbamate is a functional group with the general formula and structure . It is the analog of a carbamate in which both oxygen atoms are replaced by sulfur atoms (when only 1 oxygen is replaced the result is thioca ...
ligand, which are typically abbreviated R2dtc−. Many complexes are known. Several homoleptic derivatives have the formula M(R2dtc)n where n = 2 and 3.
Ligand characteristics
 Dithiocarbamates are anions. Because of the pi-donor properties of the amino substituent, the two sulfur centers show enhanced basicity. This situation is represented by the zwitterionic resonance structure that depicts a positive charge on N and negative charges on both sulfurs. This N to C pi-bonding results in partial double bond character. Consequently, barriers to rotational about this bond are elevated. Another consequence of their high basicity, dithiocarbamates often stabilize complexes in uncharacteristically high oxidation state (e.g., Fe(IV), Co(IV), Ni(III), Cu(III)).
Dithiocarbamate salts are easily synthesized. Many primary and secondary
Dithiocarbamates are anions. Because of the pi-donor properties of the amino substituent, the two sulfur centers show enhanced basicity. This situation is represented by the zwitterionic resonance structure that depicts a positive charge on N and negative charges on both sulfurs. This N to C pi-bonding results in partial double bond character. Consequently, barriers to rotational about this bond are elevated. Another consequence of their high basicity, dithiocarbamates often stabilize complexes in uncharacteristically high oxidation state (e.g., Fe(IV), Co(IV), Ni(III), Cu(III)).
Dithiocarbamate salts are easily synthesized. Many primary and secondary amine
In chemistry, amines (, ) are compounds and functional groups that contain a basic nitrogen atom with a lone pair. Amines are formally derivatives of ammonia (), wherein one or more hydrogen atoms have been replaced by a substituen ...
s react with carbon disulfide
Carbon disulfide (also spelled as carbon disulphide) is a neurotoxic, colorless, volatile liquid with the formula and structure . The compound is used frequently as a building block in organic chemistry as well as an industrial and chemical non ...
and sodium hydroxide
Sodium hydroxide, also known as lye and caustic soda, is an inorganic compound with the formula NaOH. It is a white solid ionic compound consisting of sodium cations and hydroxide anions .
Sodium hydroxide is a highly caustic base and alkali ...
to form dithiocarbamate salts:
:R2NH + CS2 + NaOH → R2NCS2−Na+ + H2O
A wide variety of secondary amines give the corresponding dtc ligand. Popular amines include dimethylamine
Dimethylamine is an organic compound with the formula (CH3)2NH. This secondary amine is a colorless, flammable gas with an ammonia-like odor. Dimethylamine is commonly encountered commercially as a solution in water at concentrations up to aroun ...
(Me2NH), diethylamine
Diethylamine is an organic compound with the formula (CH3CH2)2NH. It is a secondary amine. It is a flammable, weakly alkaline liquid that is miscible with most solvents. It is a colorless liquid, but commercial samples often appear brown due to im ...
(Et2NH), and pyrrolidine
Pyrrolidine, also known as tetrahydropyrrole, is an organic compound with the molecular formula (CH2)4NH. It is a cyclic secondary amine, also classified as a saturated heterocycle. It is a colourless liquid that is miscible with water and most ...
((CH2)4NH).
;Related ligands
Dithiocarbamates are classified as derivatives of dithiocarbamic acid. Their properties as ligands resemble the conjugate bases of many related "1,1-dithioacids":
*Xanthate
150px, Sodium salt of ethyl xanthate
Xanthate usually refers to a salt with the formula (R = alkyl; M+ = Na+, K+), thus they are the metal-thioate/''O''-esters of dithiocarbonate. The name ''xanthates'' is derived from Ancient Greek ''xanthos'' ...
s, ROCS2−
* Dithiophosphates, (RO)2PS2−
* Dithiocarboxylates, RCS2−
Synthetic methods
Commonly, metal dithiocarbamates are prepared bysalt metathesis reaction
A salt metathesis reaction, sometimes called a double displacement reaction, is a chemical process involving the exchange of bonds between two reacting chemical species which results in the creation of products with similar or identical bonding a ...
s using alkali metal dithiocarbamates:
:NiCl2 + 2NaS2CNMe2 → Ni(S2CNMe2)2 + 2NaCl
A complementary method entails oxidative addition
Oxidative addition and reductive elimination are two important and related classes of reactions in organometallic chemistry. Oxidative addition is a process that increases both the oxidation state and coordination number of a metal centre. Oxidat ...
of thiuram disulfide
Thiuram disulfides are a class of organosulfur compounds with the formula (R2NCSS)2. Many examples are known, but popular ones include R = Me and Et. They are disulfides obtained by oxidation of the dithiocarbamates. These compounds are used i ...
s to low-valent metal complexes:
:Mo(CO)6 + 2 2CNMe2sub>2 → Mo(S2CNMe2)4 + 6CO
Metal amido complexes, such as tetrakis(dimethylamido)titanium, react with carbon disulfide
Carbon disulfide (also spelled as carbon disulphide) is a neurotoxic, colorless, volatile liquid with the formula and structure . The compound is used frequently as a building block in organic chemistry as well as an industrial and chemical non ...
:
:Ti(NMe2)4 + 4CS2 → Ti(S2CNMe2)4
Homoleptic complexes
 ;Bis complexes
*
;Bis complexes
* nickel bis(dimethyldithiocarbamate)
Nickel bis(dimethyldithiocarbamate) is the coordination complex on nickel and dimethyldithiocarbamate, with the formula Ni(S2CNMe2)2 (Me = methyl). It is the prototype for a large number of bis(dialkhyldithiocarbamate)s of nickel(II), which featu ...
, palladium bis(dimethyldithiocarbamate), platinum bis(dimethyldithiocarbamate), all square-planar complexes
* copper bis(diethyldithiocarbamate), a square-planar complex
;Tris complexes
* vanadium tris(diethyldithiocarbamate), an octahedral complex
* chromium tris(diethylditiocarbamate), an octahedral complex
* manganese tris(dimthylthtiocarbamate), an octahedral complex
* iron tris(diethyldithiocarbamate)
Iron tris(diethyldithiocarbamate) is the coordination complex of iron with diethyldithiocarbamate with the formula Fe(S2CNEt2)3 (Et = ethyl). It is a black solid that is soluble in organic solvents.
Synthesis, structure, bonding
Iron tris(dithio ...
, ruthenium tris(diethyldithiocarbamate), osmium tris(diethyldithiocarbamate), all octahedral complexes
* cobalt tris(diethyldithiocarbamate)
Cobalt tris(diethyldithiocarbamate) is the coordination complex of cobalt with diethyldithiocarbamate with the formula Co(S2CNEt2)3 (Et = ethyl). It is a diamagnetic green solid that is soluble in organic solvents.
Synthesis, structure, bonding
C ...
, rhodium tris(diethyldithiocarbamate), iridium tris(diethyldithiocarbamate), all octahedral complexes
;Tetrakis complexes
* titanium tetrakis(dimethyldithiocarbamate)
* molybdenum tetrakis(diethyldithiocarbamate)
;Dimetallic complexes
* zinc bis(dimethyldithiocarbamate)
Zinc dimethyldithiocarbamate is a coordination complex of zinc with dimethyldithiocarbamate. It is a pale yellow solid that is used as a fungicide, the sulfur vulcanization of rubber, and other industrial applications.
Applications
Known as zir ...
, a pair of square-planar subunits
* dicobalt pentakis(diethyldithiocarbamate) cation, with a pair of octahedral Co(III) centers
* diruthenium pentakis(diethyldithiocarbamate) cation, with a pair of octahedral Ru(III) centers, two isomers
Applications
Dtc complexes find several applications: *herbicide
Herbicides (, ), also commonly known as weedkillers, are substances used to control undesired plants, also known as weeds.EPA. February 201Pesticides Industry. Sales and Usage 2006 and 2007: Market Estimates. Summary in press releasMain page fo ...
s in the form of the iron and zinc derivatives Ferbam
Iron tris(dimethyldithiocarbamate) is the coordination complex of iron with dimethyldithiocarbamate with the formula Fe(S2CNMe2)3 (Me = methyl). It is marketed as a fungicide.
Synthesis, structure, bonding
Iron tris(dithiocarbamate)s are typica ...
and Zineb
Zineb is the chemical compound with the formula n. Structurally, it is classified as a coordination polymer and a dithiocarbamate complex. This pale yellow solid is used as fungicide.
Production and applications
It is produced by treating ethyl ...
, respectively
* vulcanization accelerator
Sulfur vulcanization is a chemical process for converting natural rubber or related polymers into materials of varying hardness, elasticity, and mechanical durability by heating them with sulfur or sulfur-containing compounds. Sulfur forms cros ...
s, zinc bis(dimethyldithiocarbamate)
Zinc dimethyldithiocarbamate is a coordination complex of zinc with dimethyldithiocarbamate. It is a pale yellow solid that is used as a fungicide, the sulfur vulcanization of rubber, and other industrial applications.
Applications
Known as zir ...
* medicine, iron tris(dimethyldithiocarbamate)
Iron tris(dimethyldithiocarbamate) is the coordination complex of iron with dimethyldithiocarbamate with the formula Fe(S2CNMe2)3 (Me = methyl). It is marketed as a fungicide.
Synthesis, structure, bonding
Iron tris(dithiocarbamate)s are typica ...
as a nitric oxide
Nitric oxide (nitrogen oxide or nitrogen monoxide) is a colorless gas with the formula . It is one of the principal oxides of nitrogen. Nitric oxide is a free radical: it has an unpaired electron, which is sometimes denoted by a dot in its che ...
scavenger.
References
{{Coordination complexes Dithiocarbamates