|
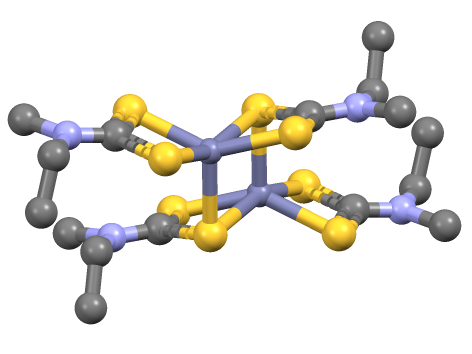
Nickel Bis(dimethyldithiocarbamate)
Nickel bis(dimethyldithiocarbamate) is the coordination complex on nickel and dimethyldithiocarbamate, with the formula Ni(S2CNMe2)2 (Me = methyl). It is the prototype for a large number of bis(dialkhyldithiocarbamate)s of nickel(II), which feature diverse organic substituents, all of which have similar structures. Nickel bis(dimethyldithiocarbamate) has been marketed as a fungicide and related complexes are used as stabilizers in polymers. Preparation and structure The compound precipitates as a black solid upon combining aqueous solutions of salts of nickel(II) and dimethyldithiocarbamate. In terms of structure and bonding the nickel is square planar, and the complex is diamagnetic.{{cite journal, title=The Chemistry of the Dithioacid and 1,1-Dithiolate Complexes, journal=Progress in Inorganic Chemistry, volume=11, author=D. Coucouvanis, pages=233–371, doi=10.1002/9780470166123.ch4 See also * Zinc dimethyldithiocarbamate - a related compound where nickel has been replaced w ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Coordination Complex
A coordination complex consists of a central atom or ion, which is usually metallic and is called the ''coordination centre'', and a surrounding array of bound molecules or ions, that are in turn known as ''ligands'' or complexing agents. Many metal-containing compounds, especially those that include transition metals (elements like titanium that belong to the Periodic Table's d-block), are coordination complexes. Nomenclature and terminology Coordination complexes are so pervasive that their structures and reactions are described in many ways, sometimes confusingly. The atom within a ligand that is bonded to the central metal atom or ion is called the donor atom. In a typical complex, a metal ion is bonded to several donor atoms, which can be the same or different. A polydentate (multiple bonded) ligand is a molecule or ion that bonds to the central atom through several of the ligand's atoms; ligands with 2, 3, 4 or even 6 bonds to the central atom are common. These compl ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Dimethyldithiocarbamate
Dimethyldithiocarbamate is the organosulfur anion with the formula (CH3)2NCS2−. It is one of the simplest organic dithiocarbamate. Uses It is a component of various pesticides and rubber chemicals in the form of its salts sodium dimethyldithiocarbamate, and potassium dimethyldithiocarbamate) as well as its complexes zinc dimethyldithiocarbamate, ferric dimethyldithiocarbamate, and nickel bis(dimethyldithiocarbamate). Oxidation gives thiram Thiram is the simplest thiuram disulfide and the oxidized dimer of dimethyldithiocarbamate. It is used as a fungicide, ectoparasiticide to prevent fungal diseases in seed and crops and similarly as an animal repellent to protect fruit trees and o .... 222px, Iron tris(dimethyldithiocarbamate) (Fe(S2CNMe2)3) is illustrative of hundreds of known dithiocarbamate complexes. References Dithiocarbamates Pesticides {{organic-compound-stub ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Methyl
In organic chemistry, a methyl group is an alkyl derived from methane, containing one carbon atom bonded to three hydrogen atoms, having chemical formula . In formulas, the group is often abbreviated as Me. This hydrocarbon group occurs in many organic compounds. It is a very stable group in most molecules. While the methyl group is usually part of a larger molecule, bounded to the rest of the molecule by a single covalent bond (), it can be found on its own in any of three forms: methanide anion (), methylium cation () or methyl radical (). The anion has eight valence electrons, the radical seven and the cation six. All three forms are highly reactive and rarely observed. Methyl cation, anion, and radical Methyl cation The methylium cation () exists in the gas phase, but is otherwise not encountered. Some compounds are considered to be sources of the cation, and this simplification is used pervasively in organic chemistry. For example, protonation of methanol gives an elect ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Fungicide
Fungicides are biocidal chemical compounds or biological organisms used to kill parasitic fungi or their spores. A fungistatic inhibits their growth. Fungi can cause serious damage in agriculture, resulting in critical losses of yield, quality, and profit. Fungicides are used both in agriculture and to fight fungal infections in animals. Chemicals used to control oomycetes, which are not fungi, are also referred to as fungicides, as oomycetes use the same mechanisms as fungi to infect plants. Fungicides can either be contact, translaminar or systemic. Contact fungicides are not taken up into the plant tissue and protect only the plant where the spray is deposited. Translaminar fungicides redistribute the fungicide from the upper, sprayed leaf surface to the lower, unsprayed surface. Systemic fungicides are taken up and redistributed through the xylem vessels. Few fungicides move to all parts of a plant. Some are locally systemic, and some move upwardly. Most fungicides that can ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Square Planar
The square planar molecular geometry in chemistry describes the stereochemistry (spatial arrangement of atoms) that is adopted by certain chemical compounds. As the name suggests, molecules of this geometry have their atoms positioned at the corners. Examples Numerous compounds adopt this geometry, examples being especially numerous for transition metal complexes. The noble gas compound XeF4 adopts this structure as predicted by VSEPR theory. The geometry is prevalent for transition metal complexes with d8 configuration, which includes Rh(I), Ir(I), Pd(II), Pt(II), and Au(III). Notable examples include the anticancer drugs cisplatin tCl2(NH3)2and carboplatin. Many homogeneous catalysts are square planar in their resting state, such as Wilkinson's catalyst and Crabtree's catalyst. Other examples include Vaska's complex and Zeise's salt. Certain ligands (such as porphyrins) stabilize this geometry. Splitting of d-orbitals A general d-orbital splitting diagram for square planar ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Zinc Dimethyldithiocarbamate
Zinc dimethyldithiocarbamate is a coordination complex of zinc with dimethyldithiocarbamate. It is a pale yellow solid that is used as a fungicide, the sulfur vulcanization of rubber, and other industrial applications. Applications Known as ziram in agriculture, it was introduced in the United States in 1960 as a broad-spectrum fungicide. It was used to address scab on apples and pears, leaf curl in peaches, and anthracnose and blight in tomatoes. In 1981, additional uses for ziram were approved, including the prevention of leaf blight and scab on almonds, shot-hole in apricots, brown rot and leaf spot in cherries, and scab and anthracnose in pecans. Ziram also began to be used on residential ornaments as a bird and mammal repellent. As a protectant fungicide, it active on the plant’s surface where it forms a chemical barrier between the plant and a fungus. A protectant fungicide is not absorbed into the plant and must be applied prior to infection. Ziram can either be direct ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Iron Tris(dimethyldithiocarbamate)
Iron tris(dimethyldithiocarbamate) is the coordination complex of iron with dimethyldithiocarbamate with the formula Fe(S2CNMe2)3 (Me = methyl). It is marketed as a fungicide. Synthesis, structure, bonding Iron tris(dithiocarbamate)s are typically are prepared by salt metathesis reactions. Iron tris(dimethyldithiocarbamate) is an octahedral coordination complex of iron(III) with D3 symmetry. Spin crossover (SCO) was first observed in 1931 by Cambi ''et al.'' who discovered anomalous magnetic behavior for the tris(N,N-dialkyldithiocarbamatoiron(III) complexes. The spin states of these complexes are sensitive to the nature of the amine substituents. Iron tris(dithiocarbamate)s characteristically react with nitric oxide to give Fe(dtc)2NO. This efficient chemical trapping reaction provides a means to detect NO. Reflecting the strongly donating properties of dithiocarbamate ligands, iron tris(dithiocarbamate)s oxidize at relatively mild potentials to give isolable iron(IV) deri ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Fungicides
Fungicides are biocidal chemical compounds or biological organisms used to kill parasitic fungi or their spores. A fungistatic inhibits their growth. Fungi can cause serious damage in agriculture, resulting in critical losses of yield, quality, and profit. Fungicides are used both in agriculture and to fight fungal infections in animals. Chemicals used to control oomycetes, which are not fungi, are also referred to as fungicides, as oomycetes use the same mechanisms as fungi to infect plants. Fungicides can either be contact, translaminar or systemic. Contact fungicides are not taken up into the plant tissue and protect only the plant where the spray is deposited. Translaminar fungicides redistribute the fungicide from the upper, sprayed leaf surface to the lower, unsprayed surface. Systemic fungicides are taken up and redistributed through the xylem vessels. Few fungicides move to all parts of a plant. Some are locally systemic, and some move upwardly. Most fungicides that can ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Nickel Complexes
Nickel is a chemical element with symbol Ni and atomic number 28. It is a silvery-white lustrous metal with a slight golden tinge. Nickel is a hard and ductile transition metal. Pure nickel is chemically reactive but large pieces are slow to react with air under standard conditions because a passivation layer of nickel oxide forms on the surface that prevents further corrosion. Even so, pure native nickel is found in Earth's crust only in tiny amounts, usually in ultramafic rocks, and in the interiors of larger nickel–iron meteorites that were not exposed to oxygen when outside Earth's atmosphere. Meteoric nickel is found in combination with iron, a reflection of the origin of those elements as major end products of supernova nucleosynthesis. An iron–nickel mixture is thought to compose Earth's outer and inner cores. Use of nickel (as natural meteoric nickel–iron alloy) has been traced as far back as 3500 BCE. Nickel was first isolated and classified as an ele ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |