|
Diazonium
Diazonium compounds or diazonium salts are a group of organic compounds sharing a common functional group where R can be any organic group, such as an alkyl or an aryl, and X is an inorganic or organic anion, such as a halide. General properties and reactivity Arenediazonium cations and related species According to X-ray crystallography the linkage is linear in typical diazonium salts. The bond distance in benzenediazonium tetrafluoroborate is 1.083(3) Å, which is almost identical to that for dinitrogen molecule (N≡N). The linear free energy constants σm and σp indicate that the diazonium group is strongly electron-withdrawing. Thus, the diazonio-substituted phenols and benzoic acids have greatly reduced p''K''a values compared to their unsubstituted counterparts. The p''K''a of phenolic proton of 4-hydroxybenzenediazonium is 3.4, versus 9.9 for phenol itself. In other words, the diazonium group lowers the p''K''a (enhances the acidity) by a million-fold. The stabil ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Azo Coupling
In organic chemistry, an azo coupling is an organic reaction between a diazonium compound () and another aromatic compound that produces an azo compound (). In this electrophilic aromatic substitution reaction, the aryldiazonium cation is the electrophile and the activated arene is a nucleophile. In most cases, including the examples below, the diazonium compound is also aromatic. Diazotization The process of conversion of primary aromatic amines into its diazonium salt is called diazotization. Diazonium salts are important synthetic intermediates that can undergo coupling reactions to form azo dyes and electrophilic substitution reactions to introduce functional groups. Uses of the reaction Aromatic azo compounds tend to be brightly colored due to the extended conjugated systems. Many are used as dyes (see azo dye). Important azo dyes include methyl red and pigment red 170. Azo printing exploits this reaction as well. Azo coupling is also used to produce prontosil and ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Benzenediazonium Cation
Benzenediazonium tetrafluoroborate is an organic compound with the formula 6H5N2F4. It is a salt of a diazonium cation and tetrafluoroborate. It exists as a colourless solid that is soluble in polar solvents. It is the parent member of the aryldiazonium compounds, which are widely used in organic chemistry. Synthesis Diazotization of aniline in the presence of hydrochloric acid: : C6H5NH2 + HNO2 + HCl → 6H5N2l + 2 H2O The tetrafluoroborate can be obtained from crude benzenediazonium chloride by salt metathesis using tetrafluoroboric acid. : 6H5N2l + HBF4 → 6H5N2F4 + HCl The tetrafluoroborate is more stable than the chloride. Properties The diazo group (N2) can be replaced by many other groups, usually anions, giving a variety of substituted phenyl derivatives: :C6H5N2+ + Nu− → C6H5Nu + N2 These transformations are associated with many named reactions including the Schiemann reaction, Sandmeyer reaction, and Gomberg-Bachmann reaction. A wide range of groups th ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Benzenediazonium Tetrafluoroborate
Benzenediazonium tetrafluoroborate is an organic compound with the formula 6H5N2F4. It is a salt of a diazonium cation and tetrafluoroborate. It exists as a colourless solid that is soluble in polar solvents. It is the parent member of the aryl diazonium compounds, which are widely used in organic chemistry. Synthesis Diazotization of aniline in the presence of hydrochloric acid: : C6H5NH2 + HNO2 + HCl → 6H5N2l + 2 H2O The tetrafluoroborate can be obtained from crude benzenediazonium chloride by salt metathesis using tetrafluoroboric acid. : 6H5N2l + HBF4 → 6H5N2F4 + HCl The tetrafluoroborate is more stable than the chloride. Properties The diazo group (N2) can be replaced by many other groups, usually anions, giving a variety of substituted phenyl derivatives: :C6H5N2+ + Nu− → C6H5Nu + N2 These transformations are associated with many named reactions including the Schiemann reaction, Sandmeyer reaction, and Gomberg-Bachmann reaction. A wide range of groups t ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Aniline
Aniline is an organic compound with the formula C6 H5 NH2. Consisting of a phenyl group attached to an amino group, aniline is the simplest aromatic amine In organic chemistry, an aromatic amine is an organic compound consisting of an aromatic ring attached to an amine. It is a broad class of compounds that encompasses aniline Aniline is an organic compound with the formula C6 H5 NH2. Consi .... It is an industrially significant Commodity chemicals, commodity chemical, as well as a versatile starting material for fine chemical synthesis. Its main use is in the manufacture of precursors to polyurethane, dyes, and other industrial chemicals. Like most volatile amines, it has the odor of rotten fish. It Combustion, ignites readily, burning with a smoky flame characteristic of aromatic compounds. It is toxic to humans. Relative to benzene, it is electron-rich. It thus participates more rapidly in electrophilic aromatic substitution reactions. Likewise, it is also prone ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Nitrous Acid
Nitrous acid (molecular formula ) is a weak and monoprotic acid known only in Solution (chemistry), solution, in the gas phase and in the form of nitrite () salts. Nitrous acid is used to make diazonium salts from amines. The resulting diazonium salts are reagents in azo coupling reactions to give azo dyes. Structure In the gas phase, the planar nitrous acid molecule can adopt both a ''syn'' and an ''anti'' form. The ''anti'' form predominates at room temperature, and infrared spectroscopy, IR measurements indicate it is Gibbs free energy, more stable by around 2.3 kJ/mol. p. 462. Image:Trans-nitrous-acid-2D-dimensions.png , Dimensions of the ''anti'' form(from the rotational spectroscopy, microwave spectrum) Image:Trans-nitrous-acid-3D-balls.png , ball-and-stick model, Model of the ''anti'' form Image:Cis-nitrous-acid-3D-balls.png , ''syn'' form Preparation Nitrous acid is usually generated by acidification of aqueous solutions of sodium nitrite with a mineral acid ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
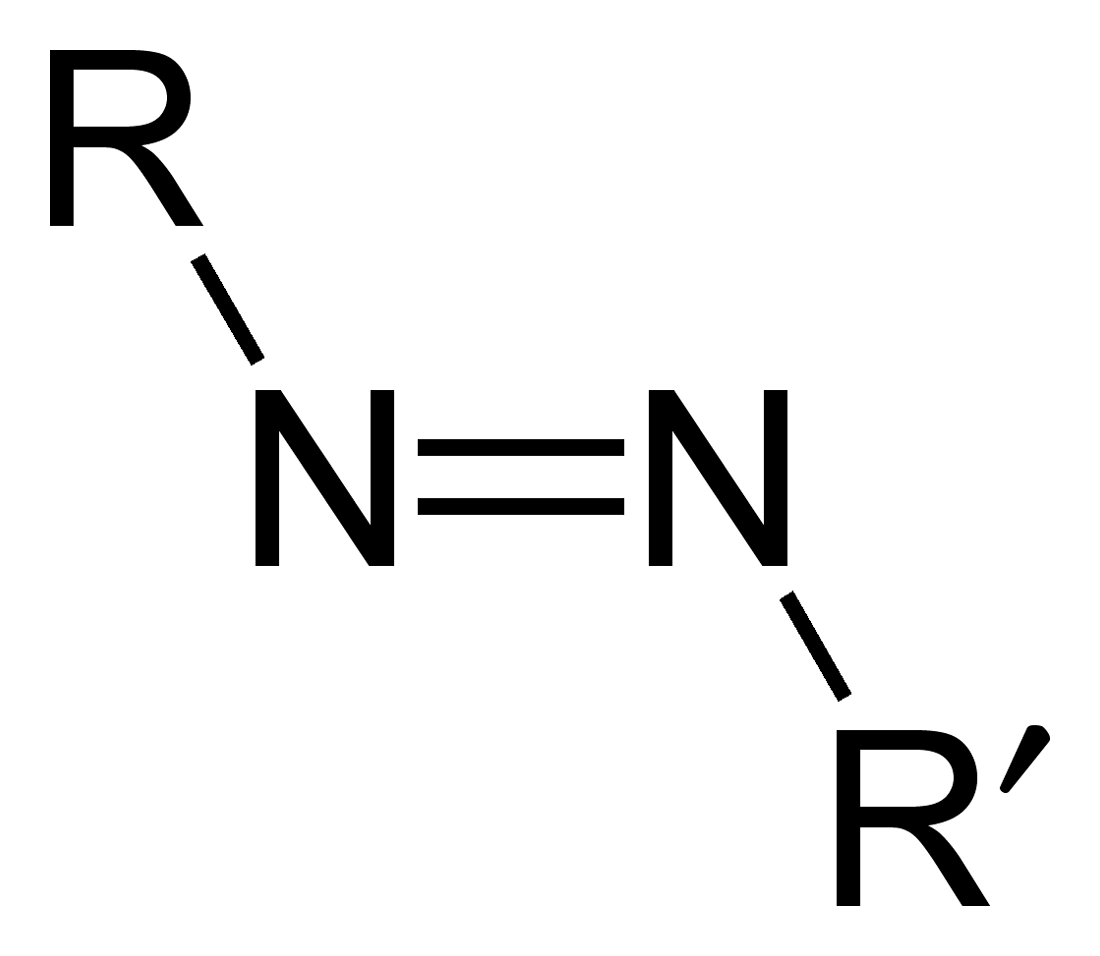
Azo Compound
Azo compounds are organic compounds bearing the functional group diazenyl (, in which R and R′ can be either aryl or alkyl groups). IUPAC defines azo compounds as: "Derivatives of diazene (diimide), , wherein both hydrogens are substituted by hydrocarbyl groups, e.g. azobenzene or diphenyldiazene." The more stable derivatives contain two aryl groups. The group is called an ''azo group'' (, ). Many textile and leather articles are dyed with azo dyes and pigments. Aryl azo compounds Aryl azo compounds are usually stable, crystalline species. Azobenzene is the prototypical aromatic azo compound. It exists mainly as the ''trans'' isomer, but upon illumination, converts to the ''cis'' isomer. Aromatic azo compounds can be synthesized by azo coupling, which entails an electrophilic substitution reaction where an aryl diazonium cation is attacked by another aryl ring, especially those substituted with electron-donating groups: :ArN2+ + Ar'H -> ArN=NAr' + H+ Since diazoniu ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Diarylide Pigment
Diarylide pigments are organic compounds that are used as pigments in inks and related materials. They often are yellow or yellow-green. To some extent, these organic compounds have displaced cadmium sulfide from the market. They exist as yellow powders of low solubility in water.K. Hunger. W. Herbst "Pigments, Organic" in ''Ullmann's Encyclopedia of Industrial Chemistry'', Wiley-VCH, Weinheim, 2012. Production The formation of these pigments involves the reaction of diazotized aromatic diamines (derivatives of benzidine) with coupling components, typically derivatives of acetoacetanilide. By varying both of these components, a wide variety of pigments have been produced. A related family of organic pigments are the simpler arylides, which arise from the coupling of monodiazonium salts with the same coupling partners. The molecular structures of these molecules is more conjugated than represented in the images shown below. The pigments' colors can range from yellow to ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Electrophilic Aromatic Substitution
Electrophilic aromatic substitution is an organic reaction in which an atom that is attached to an aromatic system (usually hydrogen) is replaced by an electrophile. Some of the most important electrophilic aromatic substitutions are aromatic nitration, aromatic halogenation, aromatic sulfonation, and alkylation and acylation Friedel–Crafts reaction. Illustrative reactions The most widely practised example of this reaction is the ethylation of benzene. :: Approximately 24,700,000 tons were produced in 1999. (After dehydrogenation and polymerization, the commodity plastic polystyrene is produced.) In this process, acids are used as catalyst to generate the incipient carbocation. Many other electrophilic reactions of benzene are conducted, although on a much smaller scale; they are valuable routes to key intermediates. The nitration of benzene is achieved via the action of the nitronium ion as the electrophile. The sulfonation with fuming sulfuric acid gives benzenesulfonic ac ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Azo Dye
Azo dyes are organic compounds bearing the functional group R−N=N−R′, in which R and R′ are usually aryl and substituted aryl groups. They are a commercially important family of azo compounds, i.e. compounds containing the C-N=N-C linkage. Azo dyes are synthetic dyes and do not occur naturally. Most azo dyes contain only one azo group, but some dyes called "disazo dyes" contain two azo groups, some dyes called "trisazo dyes" contain three azo groups and are or more. Azo dyes comprise 60-70% of all dyes used in food and textile industries. Azo dyes are widely used to treat textiles, leather articles, and some foods. Chemically related derivatives of azo dyes include azo pigments, which are insoluble in water and other solvents. Classes Many kinds of azo dyes are known, and several classification systems exist. Some classes include disperse dyes, metal-complex dyes, reactive dyes, and substantive dyes. Also called direct dyes, substantive dyes are employed for ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Diazomethane
Diazomethane is the chemical compound CH2N2, discovered by German chemist Hans von Pechmann in 1894. It is the simplest diazo compound. In the pure form at room temperature, it is an extremely sensitive explosive yellow gas; thus, it is almost universally used as a solution in diethyl ether. The compound is a popular methylating agent in the laboratory, but it is too hazardous to be employed on an industrial scale without special precautions. Use of diazomethane has been significantly reduced by the introduction of the safer and equivalent reagent trimethylsilyldiazomethane. Use For safety and convenience diazomethane is always prepared as needed as a solution in ether and used as such. It converts carboxylic acids to methyl esters and phenols into their methyl ethers. The reaction is thought to proceed via proton transfer from carboxylic acid to diazomethane to give methyldiazonium cation, which reacts with the carboxylate ion to give the methyl ester and nitrogen gas. Label ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Peter Griess
Johann Peter Griess (6 September 1829 – 30 August 1888) was an industrial chemist and an early pioneer of organic chemistry. Griess was influential in the formation of modern dyes, first formulating the diazotization reaction of arylamines. Life After he finished at an agricultural private school, he joined the Hessian cavalry, but left the military shortly after. He started his studies at the University of Jena in 1850, but changed to the University of Marburg in 1851. During his student life he was several times sentenced to the Karzer (campus jail) and was also banned from the city for one year, during which time he listened to lectures of Justus Liebig at the Ludwig Maximilian University of Munich. After most of the family possession had been spent, he had to start working at the chemical factory of Oehler in Offenbach am Main in 1856. This was only possible after the recommendation of Hermann Kolbe, who was head of the chemistry department in Marburg. The devastating fire ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Aryl
In organic chemistry, an aryl is any functional group or substituent derived from an aromatic ring, usually an aromatic hydrocarbon, such as phenyl and naphthyl. "Aryl" is used for the sake of abbreviation or generalization, and "Ar" is used as a placeholder for the aryl group in chemical structure diagrams, analogous to “R” used for any organic substituent. “Ar” is not to be confused with the elemental symbol for argon. A simple aryl group is phenyl (), a group derived from benzene. Examples of other aryl groups consist of: * The tolyl group () which is derived from toluene (methylbenzene) * The xylyl group (), which is derived from xylene (dimethylbenzene) * The naphthyl group (), which is derived from naphthalene Arylation is the process in which an aryl group is attached to a substituent. It is typically achieved by cross-coupling reactions. Nomenclature The most basic aryl group is phenyl, which is made up of a benzene ring with one hydrogen atom substituted ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |