diazomethane on:
[Wikipedia]
[Google]
[Amazon]
Diazomethane is the chemical compound CH2N2, discovered by German chemist
 In more specialized applications, diazomethane and homologues are used in Arndt-Eistert synthesis and the Büchner–Curtius–Schlotterbeck reaction for homologation.
In more specialized applications, diazomethane and homologues are used in Arndt-Eistert synthesis and the Büchner–Curtius–Schlotterbeck reaction for homologation.
 Diazomethane reacts with alcohols or
Diazomethane reacts with alcohols or
 CH2N2 reacts with basic solutions of D2O to give the deuterated derivative CD2N2.
The concentration of CH2N2 can be determined in either of two convenient ways. It can be treated with an excess of
CH2N2 reacts with basic solutions of D2O to give the deuterated derivative CD2N2.
The concentration of CH2N2 can be determined in either of two convenient ways. It can be treated with an excess of
MSDS diazomethaneSigmaaldrich technical bulletin
(PDF)
diazomethane applications and commercial availability of (Diazald) precursor
*[http://littlemsandsailing.wordpress.com/2011/05/01/identification-of-artifacts-in-diazoalkane-derivatization-reactions/ Identification of Artifacts (By-Products) in Diazomethane and Trimethylsilyldiazomethane Reactions] {{Nitrogen compounds Diazo compounds Methylating agents IARC Group 3 carcinogens Reagents for organic chemistry Explosive chemicals 1894 in science Gases with color Explosive gases Organic compounds with 1 carbon atom
Hans von Pechmann
Hans von Pechmann (1 April 1850 – 19 April 1902) was a German chemist, renowned for his discovery of diazomethane in 1894. Pechmann condensation and Pechmann pyrazole synthesis. He also first prepared 1,2-diketones (e.g., diacetyl), acetonedic ...
in 1894. It is the simplest diazo compound
The diazo group is an organic moiety consisting of two linked nitrogen atoms ( azo) at the terminal position. Overall charge neutral organic compounds containing the diazo group bound to a carbon atom are called diazo compounds or diazoalkanes ...
. In the pure form at room temperature, it is an extremely sensitive explosive yellow gas
Gas is one of the four fundamental states of matter (the others being solid, liquid, and plasma).
A pure gas may be made up of individual atoms (e.g. a noble gas like neon), elemental molecules made from one type of atom (e.g. oxygen), or ...
; thus, it is almost universally used as a solution in diethyl ether
Diethyl ether, or simply ether, is an organic compound in the ether class with the formula , sometimes abbreviated as (see Pseudoelement symbols). It is a colourless, highly volatile, sweet-smelling ("ethereal odour"), extremely flammable liq ...
. The compound is a popular methylating agent
In the chemical sciences, methylation denotes the addition of a methyl group on a substrate, or the substitution of an atom (or group) by a methyl group. Methylation is a form of alkylation, with a methyl group replacing a hydrogen atom. These t ...
in the laboratory, but it is too hazardous to be employed on an industrial scale without special precautions. Use of diazomethane has been significantly reduced by the introduction of the safer and equivalent reagent trimethylsilyldiazomethane
Trimethylsilyldiazomethane is the organosilicon compound with the formula (CH3)3SiCHN2. It is classified as a diazo compound. Trimethylsilyldiazomethane is a commercially available reagent used in organic chemistry as a methylating agent and as ...
.
Use
For safety and convenience diazomethane is always prepared as needed as a solution inether
In organic chemistry, ethers are a class of compounds that contain an ether group—an oxygen atom connected to two alkyl or aryl groups. They have the general formula , where R and R′ represent the alkyl or aryl groups. Ethers can again be c ...
and used as such. It converts carboxylic acids to methyl esters and phenols
In organic chemistry, phenols, sometimes called phenolics, are a class of chemical compounds consisting of one or more hydroxyl groups (— O H) bonded directly to an aromatic hydrocarbon group. The simplest is phenol, . Phenolic compounds are c ...
into their methyl
In organic chemistry, a methyl group is an alkyl derived from methane, containing one carbon atom bonded to three hydrogen atoms, having chemical formula . In formulas, the group is often abbreviated as Me. This hydrocarbon group occurs in many ...
ether
In organic chemistry, ethers are a class of compounds that contain an ether group—an oxygen atom connected to two alkyl or aryl groups. They have the general formula , where R and R′ represent the alkyl or aryl groups. Ethers can again be c ...
s. The reaction is thought to proceed via proton transfer from carboxylic acid to diazomethane to give methyldiazonium cation, which reacts with the carboxylate ion to give the methyl ester and nitrogen gas. Labeling studies indicate that the initial proton transfer is faster than the methyl transfer step. Since proton transfer is required for the reaction to proceed, this reaction is selective for the more acidic carboxylic acids (p''K''a ~ 5) and phenols (p''K''a ~ 10) over aliphatic alcohols (p''K''a ~ 15).
 In more specialized applications, diazomethane and homologues are used in Arndt-Eistert synthesis and the Büchner–Curtius–Schlotterbeck reaction for homologation.
In more specialized applications, diazomethane and homologues are used in Arndt-Eistert synthesis and the Büchner–Curtius–Schlotterbeck reaction for homologation.
phenol
Phenol (also called carbolic acid) is an aromatic organic compound with the molecular formula . It is a white crystalline solid that is volatile. The molecule consists of a phenyl group () bonded to a hydroxy group (). Mildly acidic, it req ...
s in presence of boron trifluoride (BF3) to give methyl
In organic chemistry, a methyl group is an alkyl derived from methane, containing one carbon atom bonded to three hydrogen atoms, having chemical formula . In formulas, the group is often abbreviated as Me. This hydrocarbon group occurs in many ...
ether
In organic chemistry, ethers are a class of compounds that contain an ether group—an oxygen atom connected to two alkyl or aryl groups. They have the general formula , where R and R′ represent the alkyl or aryl groups. Ethers can again be c ...
s.
Diazomethane is also frequently used as a carbene
In organic chemistry, a carbene is a molecule containing a neutral carbon atom with a valence of two and two unshared valence electrons. The general formula is or where the R represents substituents or hydrogen atoms.
The term "carbene" ma ...
source. It readily takes part in 1,3-dipolar cycloadditions.
Preparation
Diazomethane is prepared by hydrolysis of an ethereal solution of an ''N''-methyl nitrosamide with aqueous base. The traditional precursor is ''N''-nitroso-''N''-methylurea, but this compound is itself somewhat unstable, and nowadays compounds such as ''N''-methyl-''N-nitro-''N''-nitrosoguanidine (MNNG) and ''N''-methyl-''N''-nitroso-''p''-toluenesulfonamide (Diazald) are preferred. : CH2N2 reacts with basic solutions of D2O to give the deuterated derivative CD2N2.
The concentration of CH2N2 can be determined in either of two convenient ways. It can be treated with an excess of
CH2N2 reacts with basic solutions of D2O to give the deuterated derivative CD2N2.
The concentration of CH2N2 can be determined in either of two convenient ways. It can be treated with an excess of benzoic acid
Benzoic acid is a white (or colorless) solid organic compound with the formula , whose structure consists of a benzene ring () with a carboxyl () substituent. It is the simplest aromatic carboxylic acid. The name is derived from gum benzoin, wh ...
in cold Et2O. Unreacted benzoic acid
Benzoic acid is a white (or colorless) solid organic compound with the formula , whose structure consists of a benzene ring () with a carboxyl () substituent. It is the simplest aromatic carboxylic acid. The name is derived from gum benzoin, wh ...
is then back-titrated with standard NaOH. Alternatively, the concentration of CH2N2 in Et2O can be determined spectrophotometrically at 410 nm where its extinction coefficient, ε, is 7.2.
The gas-phase concentration of diazomethane can be determined using photoacoustic spectroscopy
Photoacoustic spectroscopy is the measurement of the effect of absorbed electromagnetic energy (particularly of light) on matter by means of acoustic detection. The discovery of the photoacoustic effect dates to 1880 when Alexander Graham Bell sh ...
.
Related compounds
Diazomethane is both isomeric andisoelectronic
Isoelectronicity is a phenomenon observed when two or more molecules have the same structure (positions and connectivities among atoms) and the same electronic configurations, but differ by what specific elements are at certain locations in th ...
with the more stable cyanamide
Cyanamide is an organic compound with the formula C N2 H2. This white solid is widely used in agriculture and the production of pharmaceuticals and other organic compounds. It is also used as an alcohol-deterrent drug. The molecule features a ...
, but they cannot interconvert.
Many substituted derivatives of diazomethane have been prepared:
*The very stable (CF3)2CN2 (2-diazo-1,1,1,3,3,3-hexafluoropropane; b.p. 12–13 °C),
*Ph2CN2 (diazodiphenylmethane
Diazodiphenylmethane is an Organic compound, organic reagent with the chemical formula C13H10N2. It exists as red-black crystals that melts just above room temperature.
Preparation
Diazodiphenylmethane can be synthesized via the oxidation of be ...
; m.p. 29–30 °C).
*(CH3)3SiCHN2 (trimethylsilyldiazomethane
Trimethylsilyldiazomethane is the organosilicon compound with the formula (CH3)3SiCHN2. It is classified as a diazo compound. Trimethylsilyldiazomethane is a commercially available reagent used in organic chemistry as a methylating agent and as ...
), which is commercially available as a solution and is as effective as CH2N2 for methylation.
* PhC(H)N2, a red liquid b.p.< 25 °C at 0.1 mm Hg.
Safety
Diazomethane is toxic by inhalation or by contact with the skin or eyes (TLV 0.2 ppm). Symptoms include chest discomfort, headache, weakness and, in severe cases, collapse.Muir, GD (ed.) 1971, ''Hazards in the Chemical Laboratory'', The Royal Institute of Chemistry, London. Symptoms may be delayed. Deaths from diazomethane poisoning have been reported. In one instance a laboratory worker consumed a hamburger near a fumehood where he was generating a large quantity of diazomethane, and died four days later from fulminating pneumonia.LeWinn, E.B. "Diazomethane Poisoning: Report of a fatal case with autopsy", ''The American Journal of the Medical Sciences'', 1949, 218, 556-562. Like any otheralkylating agent
Alkylation is the transfer of an alkyl group from one molecule to another. The alkyl group may be transferred as an alkyl carbocation, a free radical, a carbanion, or a carbene (or their equivalents). Alkylating agents are reagents for effectin ...
it is expected to be carcinogenic, but such concerns are overshadowed by its serious acute toxicity.
CH2N2 may explode in contact with sharp edges, such as ground-glass joints, even scratches in glassware. Glassware should be inspected before use and preparation should take place behind a blast shield. Specialized kits to prepare diazomethane with flame-polished joints are commercially available.
The compound explodes when heated beyond 100 °C, exposed to intense light, alkali metals, or calcium sulfate. Use of a blast shield is highly recommended while using this compound.
Proof-of-concept work has been done with microfluidics
Microfluidics refers to the behavior, precise control, and manipulation of fluids that are geometrically constrained to a small scale (typically sub-millimeter) at which surface forces dominate volumetric forces. It is a multidisciplinary field tha ...
, in which continuous point-of-use synthesis from ''N''-methyl-''N''-nitrosourea and 0.93 M potassium hydroxide in water was followed by point-of-use conversion with benzoic acid
Benzoic acid is a white (or colorless) solid organic compound with the formula , whose structure consists of a benzene ring () with a carboxyl () substituent. It is the simplest aromatic carboxylic acid. The name is derived from gum benzoin, wh ...
, resulting in a 65% yield of the methyl benzoate ester within seconds at temperatures ranging from 0 to 50 °C. The yield was better than under capillary conditions; the microfluidics were credited with "suppression of hot spots, low holdup, isothermal conditions, and intensive mixing."
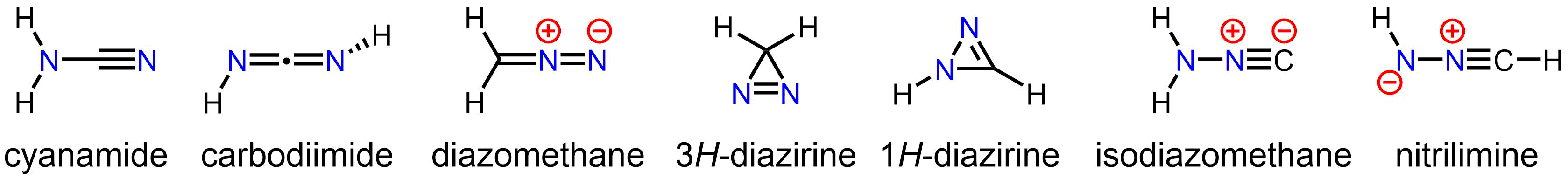
Isomers
The stable compoundcyanamide
Cyanamide is an organic compound with the formula C N2 H2. This white solid is widely used in agriculture and the production of pharmaceuticals and other organic compounds. It is also used as an alcohol-deterrent drug. The molecule features a ...
, whose minor tautomer is carbodiimide
In organic chemistry, a carbodiimide (systematic IUPAC name: methanediimine) is a functional group with the formula RN=C=NR. They are exclusively synthetic. A well known carbodiimide is dicyclohexylcarbodiimide, which is used in peptide synthesi ...
, is an isomer of diazomethane. Less stable but still isolable isomers of diazomethane include the cyclic 3''H''-diazirine and isocyanoamine (isodiazomethane
In organic chemistry, isodiazomethane, also known as isocyanamide, aminoisonitrile, or systematically as isocyanoamine, is the parent compound of a class of derivatives of general formula R2N–NC. It has the condensed formula H2N–N+≡C–, maki ...
). In addition, the parent nitrilimine Nitrilimines or nitrile amides are a class of organic compounds sharing a common functional group with the general structure R-CN-NR corresponding to the conjugate base of an amine bonded to the N-terminus of a nitrile. The dominant structure for th ...
has been observed under matrix isolation conditions.

References
External links
MSDS diazomethane
(PDF)
diazomethane applications and commercial availability of (Diazald) precursor
*[http://littlemsandsailing.wordpress.com/2011/05/01/identification-of-artifacts-in-diazoalkane-derivatization-reactions/ Identification of Artifacts (By-Products) in Diazomethane and Trimethylsilyldiazomethane Reactions] {{Nitrogen compounds Diazo compounds Methylating agents IARC Group 3 carcinogens Reagents for organic chemistry Explosive chemicals 1894 in science Gases with color Explosive gases Organic compounds with 1 carbon atom