|
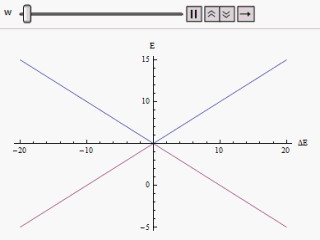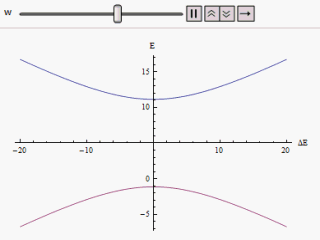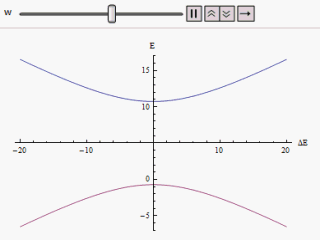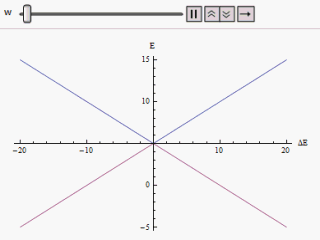
Diabatic
One of the guiding principles in modern chemical dynamics and spectroscopy is that the motion of the nuclei in a molecule is slow compared to that of its electrons. This is justified by the large disparity between the mass of an electron and the typical mass of a nucleus and leads to the Born-Oppenheimer approximation and the idea that the structure and dynamics of a chemical species are largely determined by nuclear motion on potential energy surfaces. The potential energy surfaces are obtained within the adiabatic or Born–Oppenheimer approximation. This corresponds to a representation of the molecular wave function where the variables corresponding to the molecular geometry and the electronic degrees of freedom are separated. The non separable terms are due to the nuclear kinetic energy terms in the molecular Hamiltonian and are said to couple the potential energy surfaces. In the neighbourhood of an avoided crossing or conical intersection, these terms cannot be negle ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Adiabatic Process
In thermodynamics, an adiabatic process (Greek: ''adiábatos'', "impassable") is a type of thermodynamic process that occurs without transferring heat or mass between the thermodynamic system and its environment. Unlike an isothermal process, an adiabatic process transfers energy to the surroundings only as work.. A translation may be founhere. Also a mostly reliabltranslation is to be foundin As a key concept in thermodynamics, the adiabatic process supports the theory that explains the first law of thermodynamics. Some chemical and physical processes occur too rapidly for energy to enter or leave the system as heat, allowing a convenient "adiabatic approximation".Bailyn, M. (1994), pp. 52–53. For example, the adiabatic flame temperature uses this approximation to calculate the upper limit of flame temperature by assuming combustion loses no heat to its surroundings. In meteorology and oceanography, adiabatic cooling produces condensation of moisture or salinity, overs ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Adiabatic Process (quantum Mechanics)
The adiabatic theorem is a concept in quantum mechanics. Its original form, due to Max Born and Vladimir Fock (1928), was stated as follows: :''A physical system remains in its instantaneous eigenstate if a given perturbation is acting on it slowly enough and if there is a gap between the eigenvalue and the rest of the Hamiltonian's spectrum.'' In simpler terms, a quantum mechanical system subjected to gradually changing external conditions adapts its functional form, but when subjected to rapidly varying conditions there is insufficient time for the functional form to adapt, so the spatial probability density remains unchanged. Diabatic vs. adiabatic processes At some initial time t_0 a quantum-mechanical system has an energy given by the Hamiltonian \hat(t_0); the system is in an eigenstate of \hat(t_0) labelled \psi(x,t_0). Changing conditions modify the Hamiltonian in a continuous manner, resulting in a final Hamiltonian \hat(t_1) at some later time t_1. The system will ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Vibronic Coupling
Vibronic coupling (also called nonadiabatic coupling or derivative coupling) in a molecule involves the interaction between electronic and nuclear vibrational motion. The term "vibronic" originates from the combination of the terms "vibrational" and "electronic", denoting the idea that in a molecule, vibrational and electronic interactions are interrelated and influence each other. The magnitude of vibronic coupling reflects the degree of such interrelation. In theoretical chemistry, the vibronic coupling is neglected within the Born–Oppenheimer approximation. Vibronic couplings are crucial to the understanding of nonadiabatic processes, especially near points of conical intersections. The direct calculation of vibronic couplings is not common due to difficulties associated with its evaluation. Definition Vibronic coupling describes the mixing of different electronic states as a result of small vibrations. : \mathbf_\equiv\langle\,\chi_(\mathbf;\mathbf)\,, \, \hat_\ma ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Born–Oppenheimer Approximation
In quantum chemistry and molecular physics, the Born–Oppenheimer (BO) approximation is the best-known mathematical approximation in molecular dynamics. Specifically, it is the assumption that the wave functions of atomic nuclei and electrons in a molecule can be treated separately, based on the fact that the nuclei are much heavier than the electrons. Due to the larger relative mass of a nucleus compared to an electron, the coordinates of the nuclei in a system are approximated as fixed, while the coordinates of the electrons are dynamic. The approach is named after Max Born and J. Robert Oppenheimer who proposed it in 1927, in the early period of quantum mechanics. The approximation is widely used in quantum chemistry to speed up the computation of molecular wavefunctions and other properties for large molecules. There are cases where the assumption of separable motion no longer holds, which make the approximation lose validity (it is said to "break down"), but even then ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Conical Intersection
In quantum chemistry, a conical intersection of two or more potential energy surfaces is the set of molecular geometry points where the potential energy surfaces are degenerate (intersect) and the non-adiabatic couplings between these states are non-vanishing. In the vicinity of conical intersections, the Born–Oppenheimer approximation breaks down and the coupling between electronic and nuclear motion becomes important, allowing non-adiabatic processes to take place. The location and characterization of conical intersections are therefore essential to the understanding of a wide range of important phenomena governed by non-adiabatic events, such as photoisomerization, photosynthesis, vision and the photostability of DNA. The conical intersection involving the ground electronic state potential energy surface of the C6H3F3+ molecular ion is discussed in connection with the Jahn–Teller effect in Section 13.4.2 on pages 380-388 of the textbook by Bunker and Jensen. Conical inters ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Avoided Crossing
In quantum physics and quantum chemistry, an avoided crossing (sometimes called intended crossing, ''non-crossing'' or anticrossing) is the phenomenon where two eigenvalues of an Hermitian matrix representing a quantum observable and depending on ''N'' continuous real parameters cannot become equal in value ("cross") except on a manifold of ''N''-2 dimensions. The phenomenon is also known as the von Neumann–Wigner theorem. In the case of a diatomic molecule (with one parameter, namely the bond length), this means that the eigenvalues cannot cross at all. In the case of a triatomic molecule, this means that the eigenvalues can coincide only at a single point (see conical intersection). This is particularly important in quantum chemistry. In the Born–Oppenheimer approximation, the electronic molecular Hamiltonian is diagonalized on a set of distinct molecular geometries (the obtained eigenvalues are the values of the adiabatic potential energy surfaces). The geometries for w ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Molecular Hamiltonian
In atomic, molecular, and optical physics and quantum chemistry, the molecular Hamiltonian is the Hamiltonian operator representing the energy of the electrons and nuclei in a molecule. This operator and the associated Schrödinger equation play a central role in computational chemistry and physics for computing properties of molecules and aggregates of molecules, such as thermal conductivity, specific heat, electrical conductivity, optical, and magnetic properties, and reactivity. The elementary parts of a molecule are the nuclei, characterized by their atomic numbers, ''Z'', and the electrons, which have negative elementary charge, −''e''. Their interaction gives a nuclear charge of ''Z'' + ''q'', where , with ''N'' equal to the number of electrons. Electrons and nuclei are, to a very good approximation, point charges and point masses. The molecular Hamiltonian is a sum of several terms: its major terms are the kinetic energies of the electrons and the Coulo ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Thermodynamics
Thermodynamics is a branch of physics that deals with heat, work, and temperature, and their relation to energy, entropy, and the physical properties of matter and radiation. The behavior of these quantities is governed by the four laws of thermodynamics which convey a quantitative description using measurable macroscopic physical quantities, but may be explained in terms of microscopic constituents by statistical mechanics. Thermodynamics applies to a wide variety of topics in science and engineering, especially physical chemistry, biochemistry, chemical engineering and mechanical engineering, but also in other complex fields such as meteorology. Historically, thermodynamics developed out of a desire to increase the efficiency of early steam engines, particularly through the work of French physicist Sadi Carnot (1824) who believed that engine efficiency was the key that could help France win the Napoleonic Wars. Scots-Irish physicist Lord Kelvin was the first to ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Diabetes Mellitus
Diabetes, also known as diabetes mellitus, is a group of metabolic disorders characterized by a high blood sugar level (hyperglycemia) over a prolonged period of time. Symptoms often include frequent urination, increased thirst and increased appetite. If left untreated, diabetes can cause many health complications. Acute complications can include diabetic ketoacidosis, hyperosmolar hyperglycemic state, or death. Serious long-term complications include cardiovascular disease, stroke, chronic kidney disease, foot ulcers, damage to the nerves, damage to the eyes, and cognitive impairment. Diabetes is due to either the pancreas not producing enough insulin, or the cells of the body not responding properly to the insulin produced. Insulin is a hormone which is responsible for helping glucose from food get into cells to be used for energy. There are three main types of diabetes mellitus: * Type 1 diabetes results from failure of the pancreas to produce enough insulin due ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Leibniz Rule (generalized Product Rule)
In calculus, the general Leibniz rule, named after Gottfried Wilhelm Leibniz, generalizes the product rule (which is also known as "Leibniz's rule"). It states that if f and g are n-times differentiable functions, then the product fg is also n-times differentiable and its nth derivative is given by :(fg)^=\sum_^n f^ g^, where = is the binomial coefficient and f^ denotes the ''j''th derivative of ''f'' (and in particular f^= f). The rule can be proved by using the product rule and mathematical induction. Second derivative If, for example, , the rule gives an expression for the second derivative of a product of two functions: :(fg)''(x)=\sum\limits_^=f''(x)g(x)+2f'(x)g'(x)+f(x)g''(x). More than two factors The formula can be generalized to the product of ''m'' differentiable functions ''f''1,...,''f''''m''. :\left(f_1 f_2 \cdots f_m\right)^=\sum_ \prod_f_^\,, where the sum extends over all ''m''-tuples (''k''1,...,''k''''m'') of non-negative integers with \sum_^m k_t=n, and ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Atomic Units
The Hartree atomic units are a system of natural units of measurement which is especially convenient for atomic physics and computational chemistry calculations. They are named after the physicist Douglas Hartree. By definition, the following four fundamental physical constants may each be expressed as the numeric value 1 multiplied by a coherent unit of this system: * Reduced Planck constant: \hbar, also known as the atomic unit of action * Elementary charge: e, also known as the atomic unit of charge * Bohr radius: a_0, also known as the atomic unit of length * Electron mass: m_\text, also known as the atomic unit of mass Atomic units are often abbreviated "a.u." or "au", not to be confused with the same abbreviation used also for astronomical units, arbitrary units, and absorbance units in other contexts. Defining constants Each unit in this system can be expressed as a product of powers of four physical constants without a multiplying constant. This makes it a coh ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |