|
Adiabatic Process (quantum Mechanics)
The adiabatic theorem is a concept in quantum mechanics. Its original form, due to Max Born and Vladimir Fock (1928), was stated as follows: :''A physical system remains in its instantaneous eigenstate if a given perturbation theory (quantum mechanics), perturbation is acting on it slowly enough and if there is a gap between the eigenvalue and the rest of the Hamiltonian (quantum mechanics), Hamiltonian's Spectrum of an operator, spectrum.'' In simpler terms, a quantum mechanical system subjected to gradually changing external conditions adapts its functional form, but when subjected to rapidly varying conditions there is insufficient time for the functional form to adapt, so the spatial probability density remains unchanged. Adiabatic pendulum At the 1911 Solvay conference, Einstein gave a lecture on the quantum hypothesis, which states that E = nh \nu for atomic oscillators. After Einstein's lecture, Hendrik Lorentz commented that, classically, if a simple pendulum is shorten ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Quantum Mechanics
Quantum mechanics is the fundamental physical Scientific theory, theory that describes the behavior of matter and of light; its unusual characteristics typically occur at and below the scale of atoms. Reprinted, Addison-Wesley, 1989, It is the foundation of all quantum physics, which includes quantum chemistry, quantum field theory, quantum technology, and quantum information science. Quantum mechanics can describe many systems that classical physics cannot. Classical physics can describe many aspects of nature at an ordinary (macroscopic and Microscopic scale, (optical) microscopic) scale, but is not sufficient for describing them at very small submicroscopic (atomic and subatomic) scales. Classical mechanics can be derived from quantum mechanics as an approximation that is valid at ordinary scales. Quantum systems have Bound state, bound states that are Quantization (physics), quantized to Discrete mathematics, discrete values of energy, momentum, angular momentum, and ot ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Quasistatic Process
In thermodynamics, a quasi-static process, also known as a quasi-equilibrium process (from Latin ''quasi'', meaning ‘as if’), is a thermodynamic process that happens slowly enough for the system to remain in internal physical (but not necessarily chemical) thermodynamic equilibrium. An example of this is quasi-static expansion of a mixture of hydrogen and oxygen gas, where the volume of the system changes so slowly that the pressure remains uniform throughout the system at each instant of time during the process. Such an idealized process is a succession of physical equilibrium states, characterized by infinite slowness.Rajput, R.K. (2010). ''A Textbook of Engineering Thermodynamics'', 4th edition, Laxmi Publications (P) Ltd, New Delhi, pages 21, 45, 58. Only in a quasi-static thermodynamic process can we exactly define intensive quantities (such as pressure, temperature, specific volume, specific entropy) of the system at any instant during the whole process; otherwise, ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Energy Level
A quantum mechanics, quantum mechanical system or particle that is bound state, bound—that is, confined spatially—can only take on certain discrete values of energy, called energy levels. This contrasts with classical mechanics, classical particles, which can have any amount of energy. The term is commonly used for the energy levels of the electrons in atoms, ions, or molecules, which are bound by the electric field of the atomic nucleus, nucleus, but can also refer to energy levels of nuclei or molecular vibration, vibrational or rotational energy levels in molecules. The energy spectrum of a system with such discrete energy levels is said to be Quantization (physics), quantized. In chemistry and atomic physics, an electron shell, or principal energy level, may be thought of as the orbit of one or more electrons around an atom's atomic nucleus, nucleus. The closest shell to the nucleus is called the "1 shell" (also called "K shell"), followed by the "2 shell" (or "L shell"), ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Linear Superposition
The superposition principle, also known as superposition property, states that, for all linear systems, the net response caused by two or more stimuli is the sum of the responses that would have been caused by each stimulus individually. So that if input ''A'' produces response ''X'', and input ''B'' produces response ''Y'', then input (''A'' + ''B'') produces response (''X'' + ''Y''). A function F(x) that satisfies the superposition principle is called a linear function. Superposition can be defined by two simpler properties: additivity F(x_1 + x_2) = F(x_1) + F(x_2) and homogeneity F(ax) = a F(x) for scalar . This principle has many applications in physics and engineering because many physical systems can be modeled as linear systems. For example, a beam can be modeled as a linear system where the input stimulus is the load on the beam and the output response is the deflection of the beam. The importance of linear systems is that they are easier to analyze mathemati ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Quantum Number
In quantum physics and chemistry, quantum numbers are quantities that characterize the possible states of the system. To fully specify the state of the electron in a hydrogen atom, four quantum numbers are needed. The traditional set of quantum numbers includes the principal, azimuthal, magnetic, and spin quantum numbers. To describe other systems, different quantum numbers are required. For subatomic particles, one needs to introduce new quantum numbers, such as the flavour of quarks, which have no classical correspondence. Quantum numbers are closely related to eigenvalues of observables. When the corresponding observable commutes with the Hamiltonian of the system, the quantum number is said to be " good", and acts as a constant of motion in the quantum dynamics. History Electronic quantum numbers In the era of the old quantum theory, starting from Max Planck's proposal of quanta in his model of blackbody radiation (1900) and Albert Einstein's adaptation o ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Potential Energy
In physics, potential energy is the energy of an object or system due to the body's position relative to other objects, or the configuration of its particles. The energy is equal to the work done against any restoring forces, such as gravity or those in a spring. The term ''potential energy'' was introduced by the 19th-century Scottish engineer and physicist William Rankine, although it has links to the ancient Greek philosopher Aristotle's concept of Potentiality and Actuality, ''potentiality''. Common types of potential energy include gravitational potential energy, the elastic potential energy of a deformed spring, and the electric potential energy of an electric charge and an electric field. The unit for energy in the International System of Units (SI) is the joule (symbol J). Potential energy is associated with forces that act on a body in a way that the total Work (physics), work done by these forces on the body depends only on the initial and final positions of the b ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
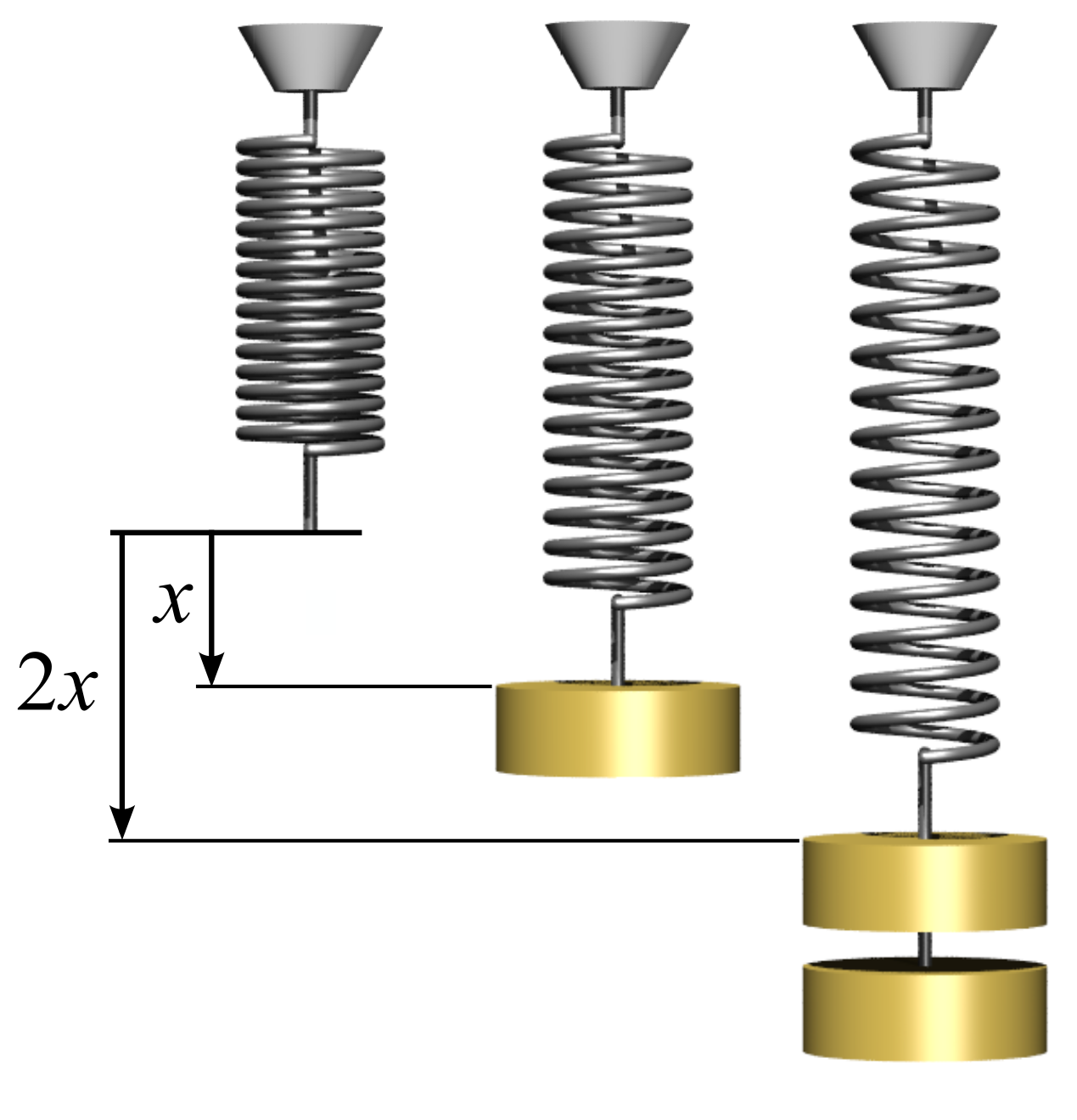
Spring Constant
In physics, Hooke's law is an empirical law which states that the force () needed to extend or compress a spring (device), spring by some distance () Proportionality (mathematics)#Direct_proportionality, scales linearly with respect to that distance—that is, where is a constant factor characteristic of the spring (i.e., its stiffness), and is small compared to the total possible deformation of the spring. The law is named after 17th-century British physicist Robert Hooke. He first stated the law in 1676 as a Latin anagram. He published the solution of his anagram in 1678 as: ("as the extension, so the force" or "the extension is proportional to the force"). Hooke states in the 1678 work that he was aware of the law since 1660. Hooke's equation holds (to some extent) in many other situations where an elasticity (physics), elastic body is Deformation (physics), deformed, such as wind blowing on a tall building, and a musician plucking a string (music), string of a guitar ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Quantum Harmonic Oscillator
The quantum harmonic oscillator is the quantum-mechanical analog of the classical harmonic oscillator. Because an arbitrary smooth potential can usually be approximated as a harmonic potential at the vicinity of a stable equilibrium point, it is one of the most important model systems in quantum mechanics. Furthermore, it is one of the few quantum-mechanical systems for which an exact, analytical solution is known. One-dimensional harmonic oscillator Hamiltonian and energy eigenstates The Hamiltonian of the particle is: \hat H = \frac + \frac k ^2 = \frac + \frac m \omega^2 ^2 \, , where is the particle's mass, is the force constant, \omega = \sqrt is the angular frequency of the oscillator, \hat is the position operator (given by in the coordinate basis), and \hat is the momentum operator (given by \hat p = -i \hbar \, \partial / \partial x in the coordinate basis). The first term in the Hamiltonian represents the kinetic energy of the particle, and the second ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Classical Physics
Classical physics refers to physics theories that are non-quantum or both non-quantum and non-relativistic, depending on the context. In historical discussions, ''classical physics'' refers to pre-1900 physics, while '' modern physics'' refers to post-1900 physics, which incorporates elements of quantum mechanics and the theory of relativity. However, relativity is based on classical field theory rather than quantum field theory, and is often categorized as a part of "classical physics". Overview ''Classical theory'' has at least two distinct meanings in physics. It can include all those areas of physics that do not make use of quantum mechanics, which includes classical mechanics (using any of the Newtonian, Lagrangian, or Hamiltonian formulations), as well as classical electrodynamics and relativity. Alternatively, the term can refer to theories that are neither quantum or relativistic. Depending on point of view, among the branches of theory sometimes included in c ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Adiabatic Invariant
A property of a physical system, such as the entropy of a gas, that stays approximately constant when changes occur slowly is called an adiabatic invariant. By this it is meant that if a system is varied between two end points, as the time for the variation between the end points is increased to infinity, the variation of an adiabatic invariant between the two end points goes to zero. In thermodynamics, an adiabatic process is a change that occurs without heat flow; it may be slow or fast. A reversible adiabatic process is an adiabatic process that occurs slowly compared to the time to reach equilibrium. In a reversible adiabatic process, the system is in equilibrium at all stages and the entropy is constant. In the 1st half of the 20th century the scientists that worked in quantum physics used the term "adiabatic" for reversible adiabatic processes and later for any gradually changing conditions which allow the system to adapt its configuration. The quantum mechanical definition i ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |