|
DIPAMP
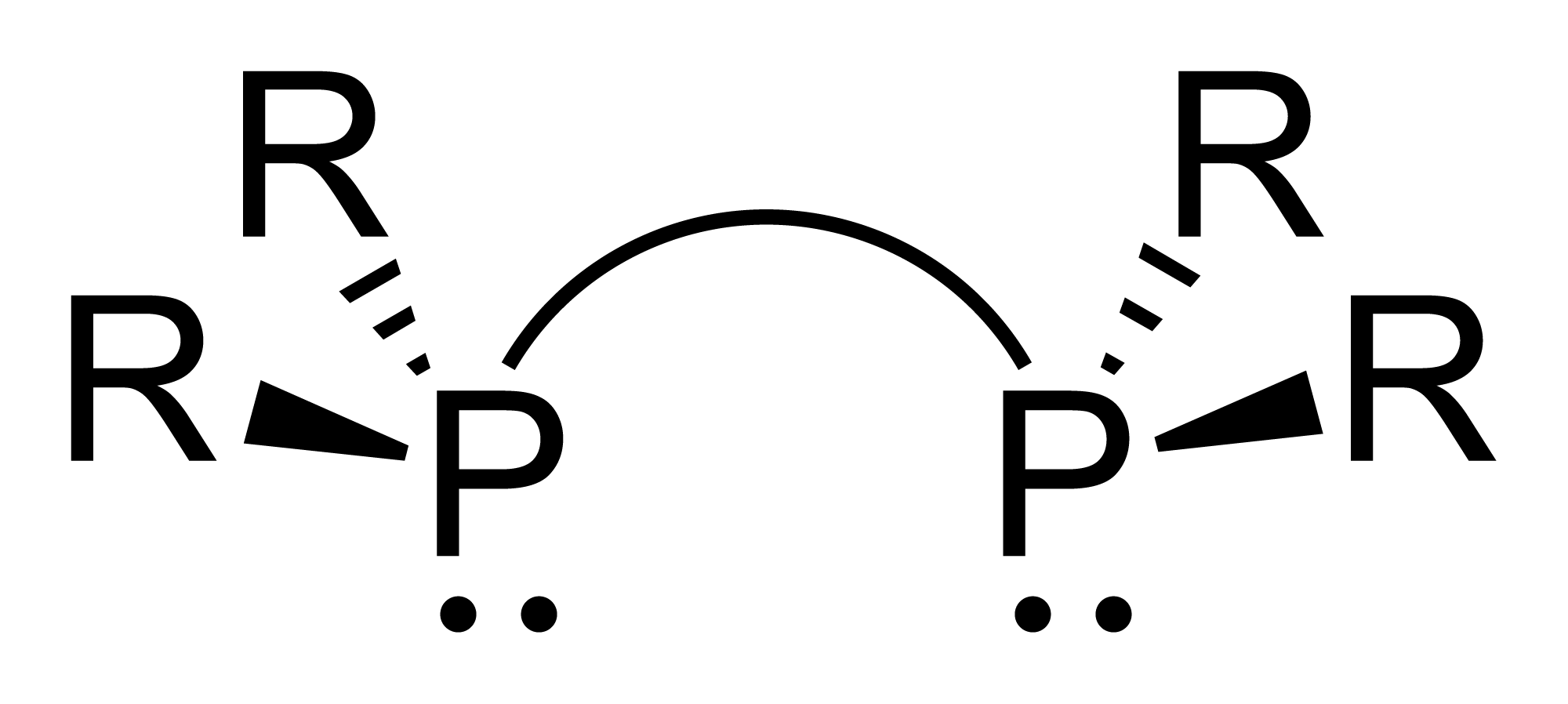
DIPAMP is an organophosphorus compound that is used as a ligand in homogeneous catalysis. It is a white solid that dissolves in organic solvents. Work on this compound by W. S. Knowles was recognized with the Nobel Prize in Chemistry. DIPAMP was the basis for of the first industrial scale asymmetric hydrogenation, the synthesis of the drug L-DOPA. : DIPAMP is a C2-symmetric diphosphine. Each phosphorus Phosphorus is a chemical element with the symbol P and atomic number 15. Elemental phosphorus exists in two major forms, white phosphorus and red phosphorus, but because it is highly reactive, phosphorus is never found as a free element on Ear ... centre, which is pyramidal, bears three different substituents - anisyl, phenyl, and the ethylene group. The ligand therefore exists as the enantiomeric (''R'',''R'') and (''S'',''S'') pair, as well as the achiral ''meso'' isomer. DIPAMP was originally prepared by an oxidative coupling, starting from anisyl(phenyl)(methyl)phosph ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Diphosphines
Diphosphines, sometimes called bisphosphanes, are organophosphorus compounds most commonly used as bidentate phosphine ligands in inorganic and organometallic chemistry. They are identified by the presence of two phosphino groups linked by a backbone, and are usually chelating. A wide variety of diphosphines have been synthesized with different linkers and R-groups. Alteration of the linker and R-groups alters the electronic and steric properties of the ligands which can result in different coordination geometries and catalytic behavior in homogeneous catalysts. Synthesis 222px, Chlorodiisopropylphosphine is a popular building block for the preparation of diphosphines. From phosphide building blocks Many widely used diphosphine ligands have the general formula Ar2P(CH2)nPAr2. These compounds can be prepared from the reaction of X(CH2)nX (X=halogen) and MPPh2 (M = alkali metal): :Cl(CH2)nCl + 2 NaPPh2 → Ph2P(CH2)nPPh2 + 2 NaCl Diphosphine ligands can also be prepare ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Diphosphines
Diphosphines, sometimes called bisphosphanes, are organophosphorus compounds most commonly used as bidentate phosphine ligands in inorganic and organometallic chemistry. They are identified by the presence of two phosphino groups linked by a backbone, and are usually chelating. A wide variety of diphosphines have been synthesized with different linkers and R-groups. Alteration of the linker and R-groups alters the electronic and steric properties of the ligands which can result in different coordination geometries and catalytic behavior in homogeneous catalysts. Synthesis 222px, Chlorodiisopropylphosphine is a popular building block for the preparation of diphosphines. From phosphide building blocks Many widely used diphosphine ligands have the general formula Ar2P(CH2)nPAr2. These compounds can be prepared from the reaction of X(CH2)nX (X=halogen) and MPPh2 (M = alkali metal): :Cl(CH2)nCl + 2 NaPPh2 → Ph2P(CH2)nPPh2 + 2 NaCl Diphosphine ligands can also be prepare ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Asymmetric Hydrogenation
Asymmetric hydrogenation is a chemical reaction that adds two atoms of Hydrogen atom, hydrogen to a target (substrate) molecule with three-dimensional Enantioselective synthesis, spatial selectivity. Critically, this selectivity does not come from the target molecule itself, but from other reagents or catalysts present in the reaction. This allows spatial information (what chemists refer to as chirality) to transfer from one molecule to the target, forming the product as a single enantiomer. The chiral information is most commonly contained in a catalyst and, in this case, the information in a single molecule of catalyst may be transferred to many substrate molecules, amplifying the amount of chiral information present. Similar processes occur in nature, where a chiral molecule like an enzyme can catalyse the introduction of a chiral centre to give a product as a single enantiomer, such as amino acids, that a cell needs to function. By imitating this process, chemists can generate ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
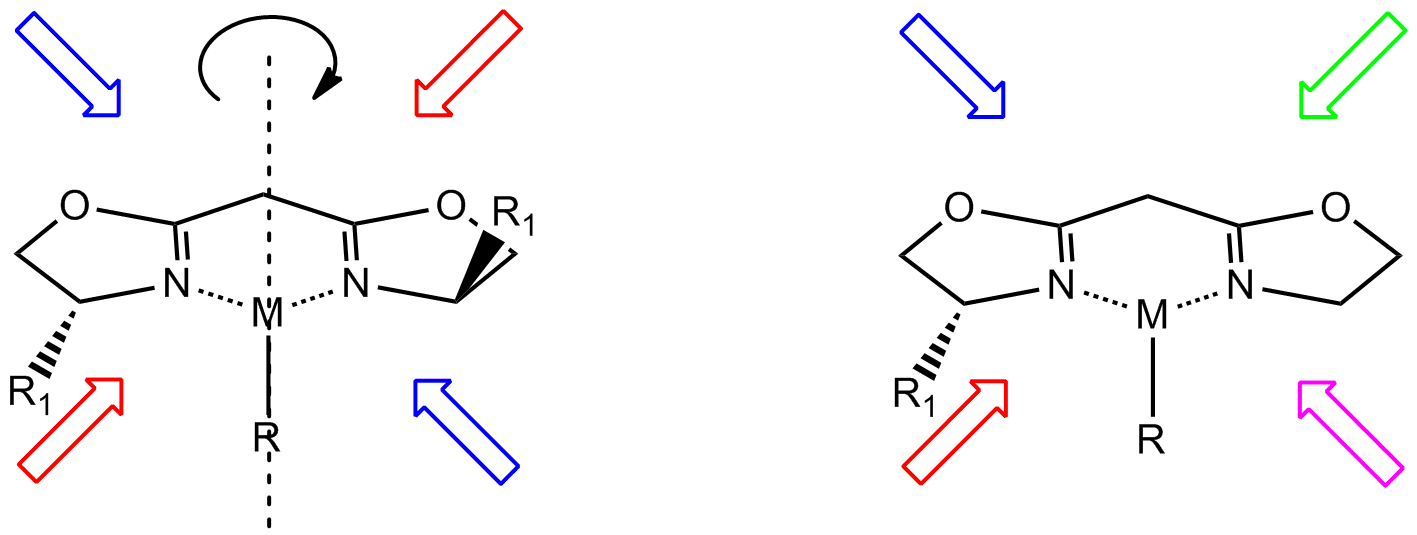
C2-Symmetric Ligands
In homogeneous catalysis, ''C''2-symmetric ligands refer to ligands that lack mirror symmetry but have ''C''2 symmetry (two-fold rotational symmetry). Such ligands are usually bidentate and are valuable in catalysis. The ''C''2 symmetry of ligands limits the number of possible reaction pathways and thereby increases enantioselectivity, relative to asymmetrical analogues. ''C''2-symmetric ligands are a subset of chiral ligands. Chiral ligands, including ''C''2-symmetric ligands, combine with metals or other groups to form chiral catalysts. These catalysts engage in enantioselective chemical synthesis, in which chirality in the catalyst yields chirality in the reaction product. Examples An early ''C''2-symmetric ligand, diphosphine catalytic ligand DIPAMP, was developed in 1968 by William S. Knowles and coworkers of Monsanto Company, who shared the 2001 Nobel Prize in Chemistry. This ligand was used in the industrial production of -DOPA. : Some classes of ''C''2-symmetric li ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Organophosphorus Compound
Organophosphorus compounds are organic compounds containing phosphorus. They are used primarily in pest control as an alternative to chlorinated hydrocarbons that persist in the environment. Some organophosphorus compounds are highly effective insecticides, although some are extremely toxic to humans, including sarin and VX nerve agents. Organophosphorus chemistry is the corresponding science of the properties and reactivity of organophosphorus compounds. Phosphorus, like nitrogen, is in group 15 of the periodic table, and thus phosphorus compounds and nitrogen compounds have many similar properties. The definition of organophosphorus compounds is variable, which can lead to confusion. In industrial and environmental chemistry, an organophosphorus compound need contain only an organic substituent, but need not have a direct phosphorus-carbon (P-C) bond. Thus a large proportion of pesticides (e.g., malathion), are often included in this class of compounds. Phosphorus can adopt ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Ligand
In coordination chemistry, a ligand is an ion or molecule (functional group) that binds to a central metal atom to form a coordination complex. The bonding with the metal generally involves formal donation of one or more of the ligand's electron pairs, often through Lewis bases. The nature of metal–ligand bonding can range from covalent to ionic. Furthermore, the metal–ligand bond order can range from one to three. Ligands are viewed as Lewis bases, although rare cases are known to involve Lewis acidic "ligands". Metals and metalloids are bound to ligands in almost all circumstances, although gaseous "naked" metal ions can be generated in a high vacuum. Ligands in a complex dictate the reactivity of the central atom, including ligand substitution rates, the reactivity of the ligands themselves, and redox. Ligand selection requires critical consideration in many practical areas, including bioinorganic and medicinal chemistry, homogeneous catalysis, and environmental chemi ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Homogeneous Catalysis
In chemistry, homogeneous catalysis is catalysis by a soluble catalyst in a solution. Homogeneous catalysis refers to reactions where the catalyst is in the same phase as the reactants, principally in solution. In contrast, heterogeneous catalysis describes processes where the catalysts and substrate are in distinct phases, typically solid-gas, respectively. The term is used almost exclusively to describe solutions and implies catalysis by organometallic compounds. Homogeneous catalysis is an established technology that continues to evolve. An illustrative major application is the production of acetic acid. Enzymes are examples of homogeneous catalysts. Examples Acid catalysis The proton is a pervasive homogeneous catalyst because water is the most common solvent. Water forms protons by the process of self-ionization of water. In an illustrative case, acids accelerate (catalyze) the hydrolysis of esters: :CH3CO2CH3 + H2O CH3CO2H + CH3OH At neutral pH, aqueous solutions of most e ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Nobel Prize In Chemistry
) , image = Nobel Prize.png , alt = A golden medallion with an embossed image of a bearded man facing left in profile. To the left of the man is the text "ALFR•" then "NOBEL", and on the right, the text (smaller) "NAT•" then "MDCCCXXXIII" above, followed by (smaller) "OB•" then "MDCCCXCVI" below. , awarded_for = Outstanding contributions in chemistry , presenter = Royal Swedish Academy of Sciences , location = Stockholm, Sweden , reward = 9 million SEK (2017) , year = 1901 , holder = Carolyn R. Bertozzi, Morten P. Meldal and Karl Barry Sharpless (2022) , most_awards = Frederick Sanger and Karl Barry Sharpless (2) , website nobelprize.org, previous = 2021 , year2=2022, main=2022, next=2023 The Nobel Prize in Chemistry is awarded annually by the Royal Swedish Academy of Sciences to scientists in the various fields of chemistry. It is one of the five Nobel Prizes established by the will of Alfred Nobel in 1895, awarded for ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
L-DOPA
-DOPA, also known as levodopa and -3,4-dihydroxyphenylalanine, is an amino acid that is made and used as part of the normal biology of some plants and animals, including humans. Humans, as well as a portion of the other animals that utilize -DOPA, make it via biosynthesis from the amino acid -tyrosine. -DOPA is the precursor to the neurotransmitters dopamine, norepinephrine (noradrenaline), and epinephrine (adrenaline), which are collectively known as catecholamines. Furthermore, -DOPA itself mediates neurotrophic factor release by the brain and CNS. -DOPA can be manufactured and in its pure form is sold as a psychoactive drug with the INN levodopa; trade names include Sinemet, Pharmacopa, Atamet, and Stalevo. As a drug, it is used in the clinical treatment of Parkinson's disease and dopamine-responsive dystonia. -DOPA has a counterpart with opposite chirality, -DOPA. As is true for many molecules, the human body produces only one of these isomers (the -DOPA form). The ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Phosphorus
Phosphorus is a chemical element with the symbol P and atomic number 15. Elemental phosphorus exists in two major forms, white phosphorus and red phosphorus, but because it is highly reactive, phosphorus is never found as a free element on Earth. It has a concentration in the Earth's crust of about one gram per kilogram (compare copper at about 0.06 grams). In minerals, phosphorus generally occurs as phosphate. Elemental phosphorus was first isolated as white phosphorus in 1669. White phosphorus emits a faint glow when exposed to oxygen – hence the name, taken from Greek mythology, meaning 'light-bearer' (Latin ), referring to the " Morning Star", the planet Venus. The term '' phosphorescence'', meaning glow after illumination, derives from this property of phosphorus, although the word has since been used for a different physical process that produces a glow. The glow of phosphorus is caused by oxidation of the white (but not red) phosphorus — a process now called chem ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Anisole
Anisole, or methoxybenzene, is an organic compound with the formula CH3OC6H5. It is a colorless liquid with a smell reminiscent of anise seed, and in fact many of its derivatives are found in natural and artificial fragrances. The compound is mainly made synthetically and is a precursor to other synthetic compounds. It is an ether. Anisole is a standard reagent of both practical and pedagogical value. It can be prepared by the Williamson ether synthesis; sodium phenoxide is reacted with a methyl halide to yield anisole. Reactivity Anisole undergoes electrophilic aromatic substitution reaction at a faster speed than benzene, which in turn reacts more quickly than nitrobenzene. The methoxy group is an ortho/para directing group, which means that electrophilic substitution preferentially occurs at these three sites. The enhanced nucleophilicity of anisole vs. benzene reflects the influence of the methoxy group, which renders the ring more electron-rich. The methoxy group str ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |

4-3D-balls.png)


