|
C2-Symmetric Ligands
In homogeneous catalysis, ''C''2-symmetric ligands refer to ligands that lack mirror symmetry but have ''C''2 symmetry (two-fold rotational symmetry). Such ligands are usually bidentate and are valuable in catalysis. The ''C''2 symmetry of ligands limits the number of possible reaction pathways and thereby increases enantioselectivity, relative to asymmetrical analogues. ''C''2-symmetric ligands are a subset of chiral ligands. Chiral ligands, including ''C''2-symmetric ligands, combine with metals or other groups to form chiral catalysts. These catalysts engage in enantioselective chemical synthesis, in which chirality in the catalyst yields chirality in the reaction product. Examples An early ''C''2-symmetric ligand, diphosphine catalytic ligand DIPAMP, was developed in 1968 by William S. Knowles and coworkers of Monsanto Company, who shared the 2001 Nobel Prize in Chemistry. This ligand was used in the industrial production of -DOPA. : Some classes of ''C''2-symmetric li ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Homogeneous Catalysis
In chemistry, homogeneous catalysis is catalysis by a soluble catalyst in a solution. Homogeneous catalysis refers to reactions where the catalyst is in the same phase as the reactants, principally in solution. In contrast, heterogeneous catalysis describes processes where the catalysts and substrate are in distinct phases, typically solid-gas, respectively. The term is used almost exclusively to describe solutions and implies catalysis by organometallic compounds. Homogeneous catalysis is an established technology that continues to evolve. An illustrative major application is the production of acetic acid. Enzymes are examples of homogeneous catalysts. Examples Acid catalysis The proton is a pervasive homogeneous catalyst because water is the most common solvent. Water forms protons by the process of self-ionization of water. In an illustrative case, acids accelerate (catalyze) the hydrolysis of esters: :CH3CO2CH3 + H2O CH3CO2H + CH3OH At neutral pH, aqueous solutions of most e ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Asymmetric Hydrogenation
Asymmetric hydrogenation is a chemical reaction that adds two atoms of Hydrogen atom, hydrogen to a target (substrate) molecule with three-dimensional Enantioselective synthesis, spatial selectivity. Critically, this selectivity does not come from the target molecule itself, but from other reagents or catalysts present in the reaction. This allows spatial information (what chemists refer to as chirality) to transfer from one molecule to the target, forming the product as a single enantiomer. The chiral information is most commonly contained in a catalyst and, in this case, the information in a single molecule of catalyst may be transferred to many substrate molecules, amplifying the amount of chiral information present. Similar processes occur in nature, where a chiral molecule like an enzyme can catalyse the introduction of a chiral centre to give a product as a single enantiomer, such as amino acids, that a cell needs to function. By imitating this process, chemists can generate ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
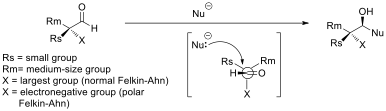
Asymmetric Induction
In stereochemistry, asymmetric induction (also enantioinduction) describes the preferential formation in a chemical reaction of one enantiomer or diastereoisomer over the other as a result of the influence of a chiral feature present in the substrate, reagent, catalyst or environment. Asymmetric induction is a key element in asymmetric synthesis. Asymmetric induction was introduced by Hermann Emil Fischer based on his work on carbohydrates. Several types of induction exist. Internal asymmetric induction makes use of a chiral center bound to the reactive center through a covalent bond and remains so during the reaction. The starting material is often derived from chiral pool synthesis. In relayed asymmetric induction the chiral information is introduced in a separate step and removed again in a separate chemical reaction. Special synthons are called chiral auxiliaries. In external asymmetric induction chiral information is introduced in the transition state through a catalyst o ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Sharpless Asymmetric Dihydroxylation
Sharpless asymmetric dihydroxylation (also called the Sharpless bishydroxylation) is the chemical reaction of an alkene with osmium tetroxide in the presence of a chiral quinine ligand to form a vicinal diol. The reaction has been applied to alkenes of virtually every substitution, often high enantioselectivities are realized, with the chiral outcome controlled by the choice of dihydroquinidine (DHQD) vs dihydroquinine (DHQ) as the ligand. Asymmetric dihydroxylation reactions are also highly site selective, providing products derived from reaction of the most electron-rich double bond in the substrate. It is common practice to perform this reaction using a catalytic amount of osmium tetroxide, which after reaction is regenerated with reoxidants such as potassium ferricyanide or ''N''-methylmorpholine ''N''-oxide. This dramatically reduces the amount of the highly toxic and very expensive osmium tetroxide needed. These four reagents are commercially available premixed (" AD- ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Dihydroquinine
Dihydroquinine, also known as hydroquinine, is an organic compound and as a cinchona alkaloid closely related to quinine. The specific rotation is −148° in ethanol. A derivative of this molecule is used as chiral ligand in the AD-mix for Sharpless dihydroxylation Sharpless asymmetric dihydroxylation (also called the Sharpless bishydroxylation) is the chemical reaction of an alkene with osmium tetroxide in the presence of a chiral quinine ligand to form a vicinal diol. The reaction has been applied to alk .... See also * Dihydroquinidine Buchler a company focussing solely on cinchona alkaloids as catalysts such as Dihydroquinine or Dihydroquinidine">Dihydroquinidine">Buchler a company focussing solely on cinchona alkaloids as catalysts such as Dihydroquinine or Dihydroquinidine References {{Other drugs for disorders of the musculo-skeletal system Secondary alcohols Phenol ethers Quinoline alkaloids Quinuclidine alkaloids ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
BINAP
BINAP (2,2′-bis(diphenylphosphino)-1,1′-binaphthyl) is an organophosphorus compound. This chiral diphosphine ligand is widely used in asymmetric synthesis. It consists of a pair of 2-diphenylphosphinonaphthyl groups linked at the 1 and 1′ positions. This C2-symmetric framework lacks a stereogenic atom, but has axial chirality due to restricted rotation ( atropisomerism). The barrier to racemization is high due to steric hindrance, which limits rotation about the bond linking the naphthyl rings. The dihedral angle between the naphthyl groups is approximately 90°. The natural bite angle is 93°. Use as ligand in asymmetric catalysis BINAP is used in organic synthesis for enantioselective transformations catalyzed by its complexes of ruthenium, rhodium, and palladium. As pioneered by Ryōji Noyori and his co-workers, rhodium complexes of BINAP are useful for the synthesis of (–)-menthol. Silver complexes are also important; BINAP- AgF can be used to enantioselect ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Organic Synthesis
Organic synthesis is a special branch of chemical synthesis and is concerned with the intentional construction of organic compounds. Organic molecules are often more complex than inorganic compounds, and their synthesis has developed into one of the most important branches of organic chemistry. There are several main areas of research within the general area of organic synthesis: ''total synthesis'', ''semisynthesis'', and ''methodology''. Total synthesis A total synthesis is the complete chemical synthesis of complex organic molecules from simple, commercially available petrochemical or natural precursors. Total synthesis may be accomplished either via a linear or convergent approach. In a ''linear'' synthesis—often adequate for simple structures—several steps are performed one after another until the molecule is complete; the chemical compounds made in each step are called synthetic intermediates. Most often, each step in a synthesis refers to a separate rea ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Salen Ligand
Salen refers to a tetradentate C2-symmetric ligand synthesized from salicylaldehyde (sal) and ethylenediamine (en). It may also refer to a class of compounds, which are structurally related to the classical salen ligand, primarily bis-Schiff bases. Salen ligands are notable for coordinating a wide range of different metals, which they can often stabilise in various oxidation states. For this reason salen-type compounds are used as metal deactivators. Metal salen complexes also find use as catalysts. Synthesis and properties H2salen may be synthesized by the condensation of ethylenediamine and salicylaldehyde. : Complexes of salen with metal cations may be made without isolating it from the reaction mixture. This is possible because the stability constant for the formation of the metal complexes are very high, due to the chelate effect. :H2L + Mn+ → ML(n−2)+ + 2 H+ where L stands for the ligand. The pyridine adduct of the cobalt(II) complex Co(salen)(py) ( salcomine) ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Jacobsen's Catalyst
Jacobsen's catalyst is the common name for N,N'-bis(3,5-di-tert-butylsalicylidene)-1,2-cyclohexanediaminomanganese(III) chloride, a coordination compound of manganese and a salen ligand, salen-type ligand. It is used as an asymmetric catalyst in the Jacobsen epoxidation, which is renowned for its ability to enantioselectively transform prochiral alkenes into epoxides. Before its development, catalysts for the asymmetric epoxidation of alkenes required the substrate to have a directing functional group, such as an alcohol as seen in the Sharpless epoxidation. This compound has two enantiomers, which give the appropriate epoxide product from the alkene starting material. Enantiomerically pure epoxides are desirable as building blocks for complex molecules with specific chirality. Biologically active compounds can exhibit radically different activity based on differences in chirality and therefore the ability to obtain desired stereocenters in a molecule is of great importance to ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |


-Jacobsen's_catalyst.png)