|
Copper(II) Tetrafluoroborate
Copper(II) tetrafluoroborate is any inorganic compound with the formula Cu(H2O)x (BF4)2. As usually encountered, it is assumed to be the hexahydrate (x = 6), but this salt can be partially dehydrated to the tetrahydrate. Regardless, these compounds are aquo complexes of copper in its +2 oxidation state, with two weakly coordinating tetrafluoroborate anions. The compound is used in organic synthesis Organic synthesis is a special branch of chemical synthesis and is concerned with the intentional construction of organic compounds. Organic molecules are often more complex than inorganic compounds, and their synthesis has developed into one o ..., e.g. as a Lewis acid for Diels Alder reactions, for cyclopropanation of alkenes with diazo reagents, and as a Lewis Acid in Meinwald Rearrangement reactions on Epoxides. In the former two applications, the copper(II) is reduced to a copper(I) catalyst.Ilhyong Ryu, Noboru Sonoda, "Copper(II) Tetrafluoroborate" Encyclopedia of R ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Copper(II) Chloride
Copper(II) chloride is the chemical compound with the chemical formula CuCl2. The anhydrous form is yellowish brown but slowly absorbs moisture to form a blue-green dihydrate. Both the anhydrous and the dihydrate forms occur naturally as the very rare minerals tolbachite and eriochalcite, respectively.Marlene C. Morris, Howard F. McMurdie, Eloise H. Evans, Boris Paretzkin, Harry S. Parker, and Nicolas C. Panagiotopoulos (1981) ''Copper chloride hydrate (eriochalcite)'', in Standard X-ray Diffraction Powder PatternsNational Bureau of Standards, Monograph 25, Section 18; page 33. Structure Anhydrous CuCl2 adopts a distorted cadmium iodide structure. In this motif, the copper centers are octahedral. Most copper(II) compounds exhibit distortions from idealized octahedral geometry due to the Jahn-Teller effect, which in this case describes the localization of one d-electron into a molecular orbital that is strongly antibonding with respect to a pair of chloride ligands. In CuCl2·2H ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Oxidation State
In chemistry, the oxidation state, or oxidation number, is the hypothetical charge of an atom if all of its bonds to different atoms were fully ionic. It describes the degree of oxidation (loss of electrons) of an atom in a chemical compound. Conceptually, the oxidation state may be positive, negative or zero. While fully ionic bonds are not found in nature, many bonds exhibit strong ionicity, making oxidation state a useful predictor of charge. The oxidation state of an atom does not represent the "real" formal charge on that atom, or any other actual atomic property. This is particularly true of high oxidation states, where the ionization energy required to produce a multiply positive ion is far greater than the energies available in chemical reactions. Additionally, the oxidation states of atoms in a given compound may vary depending on the choice of electronegativity scale used in their calculation. Thus, the oxidation state of an atom in a compound is purely a formalism. ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Copper Electroplating
Copper electroplating is the process of electroplating a layer of copper onto the surface of a metal object. Copper is used both as a standalone coating and as an undercoat onto which other metals are subsequently plated. The copper layer can be decorative, provide corrosion resistance, increase electrical and thermal conductivity, or improve the adhesion of additional deposits to the substrate. Overview Copper electroplating takes place in an electrolytic cell using electrolysis. As with all plating processes, the part to be plated must be cleaned before depositing metal to remove soils, grease, oxides, and defects. After precleaning, the part is immersed in the cell's aqueous electrolyte solution and functions as the cathode. A copper anode is also immersed in the solution. During plating, a direct electric current is applied to the cell which causes the copper in the anode to dissolve into the electrolyte through oxidation, losing electrons and ionizing into copper cations. The ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Cyclopropanation
In organic chemistry, cyclopropanation refers to any chemical process which generates cyclopropane () rings. It is an important process in modern chemistry as many useful compounds bear this motif; for example pyrethroids and a number of quinolone antibiotics (ciprofloxacin, sparfloxacin, etc.). However, the high ring strain present in cyclopropanes makes them challenging to produce and generally requires the use of highly reactive species, such as carbenes, ylids and carbanions. Many of the reactions proceed in a cheletropic manner. Approaches From alkenes using carbenoid reagents Several methods exist for converting alkenes to cyclopropane rings using carbene type reagents. As carbenes themselves are highly reactive it is common for them to be used in a stabilised form, referred to as a carbenoid. Simmons–Smith reaction In the Simmons–Smith reaction the reactive carbenoid is iodomethylzinc iodide, which is typically formed by a reaction between diiodomethane and a z ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Diels Alder Reaction (1848–1922), German classical scholar
{{surname ...
Diels is the last name of several people: * Rudolf Diels (1900–1957), German politician * Otto Diels (1876–1954), German scientist noted for his work on the Diels–Alder reaction * Ludwig Diels (1874–1945), German botanist * Hermann Diels Hermann Alexander Diels (; 18 May 1848 – 4 June 1922) was a German classical scholar, who was influential in the area of early Greek philosophy and is known for his standard work ''Die Fragmente der Vorsokratiker''. Diels helped to import the ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Organic Synthesis
Organic synthesis is a special branch of chemical synthesis and is concerned with the intentional construction of organic compounds. Organic molecules are often more complex than inorganic compounds, and their synthesis has developed into one of the most important branches of organic chemistry. There are several main areas of research within the general area of organic synthesis: ''total synthesis'', ''semisynthesis'', and ''methodology''. Total synthesis A total synthesis is the complete chemical synthesis of complex organic molecules from simple, commercially available petrochemical or natural precursors. Total synthesis may be accomplished either via a linear or convergent approach. In a ''linear'' synthesis—often adequate for simple structures—several steps are performed one after another until the molecule is complete; the chemical compounds made in each step are called synthetic intermediates. Most often, each step in a synthesis refers to a separate rea ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Anion
An ion () is an atom or molecule with a net electrical charge. The charge of an electron is considered to be negative by convention and this charge is equal and opposite to the charge of a proton, which is considered to be positive by convention. The net charge of an ion is not zero because its total number of electrons is unequal to its total number of protons. A cation is a positively charged ion with fewer electrons than protons while an anion is a negatively charged ion with more electrons than protons. Opposite electric charges are pulled towards one another by electrostatic force, so cations and anions attract each other and readily form ionic compounds. Ions consisting of only a single atom are termed atomic or monatomic ions, while two or more atoms form molecular ions or polyatomic ions. In the case of physical ionization in a fluid (gas or liquid), "ion pairs" are created by spontaneous molecule collisions, where each generated pair consists of a free electron and ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
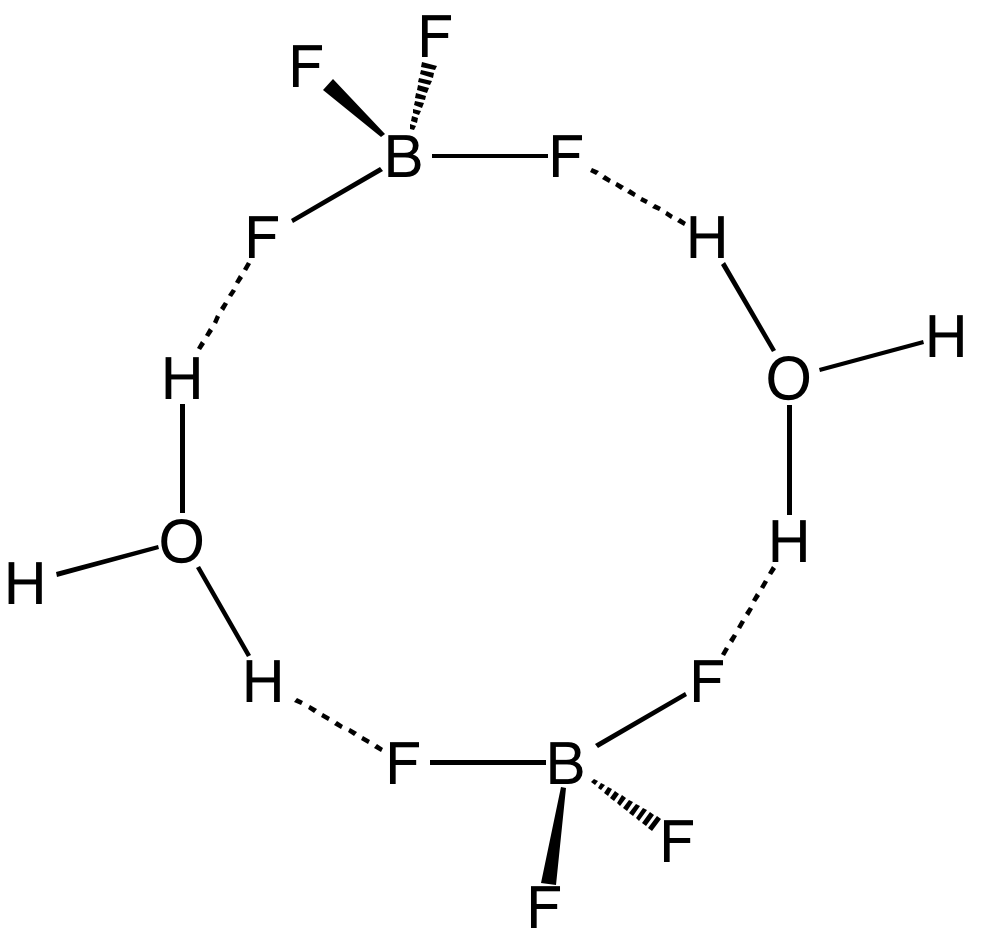
Tetrafluoroborate
Tetrafluoroborate is the anion . This tetrahedral species is isoelectronic with tetrafluoroberyllate (), tetrafluoromethane (CF4), and tetrafluoroammonium () and is valence isoelectronic with many stable and important species including the perchlorate anion, , which is used in similar ways in the laboratory. It arises by the reaction of fluoride salts with the Lewis acid BF3, treatment of tetrafluoroboric acid with base, or by treatment of boric acid with hydrofluoric acid. As an anion in inorganic and organic chemistry The popularization of has led to decreased use of in the laboratory as a weakly coordinating anion. With organic compounds, especially amine derivatives, forms potentially explosive derivatives. Disadvantages to include its slight sensitivity to hydrolysis and decomposition via loss of a fluoride ligand, whereas does not suffer from these problems. Safety considerations, however, overshadow this inconvenience. With a formula weight of 86.8, BF is also conveni ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Aquo Complex
In chemistry, metal aquo complexes are coordination compounds containing metal ions with only water as a ligand. These complexes are the predominant species in aqueous solutions of many metal salts, such as metal nitrates, sulfates, and perchlorates. They have the general stoichiometry . Their behavior underpins many aspects of environmental, biological, and industrial chemistry. This article focuses on complexes where water is the only ligand ("homoleptic aquo complexes"), but of course many complexes are known to consist of a mix of aquo and other ligands. Stoichiometry and structure Hexa-aquo complexes Most aquo complexes are mono-nuclear, with the general formula , with or 3; they have an octahedral structure. The water molecules function as Lewis bases, donating a pair of electrons to the metal ion and forming a dative covalent bond with it. Typical examples are listed in the following table. : Tutton's salts are crystalline compounds with the generic formula (where ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Copper(II) Oxide
Copper(II) oxide or cupric oxide is an inorganic compound with the formula CuO. A black solid, it is one of the two stable oxides of copper, the other being Cu2O or copper(I) oxide (cuprous oxide). As a mineral, it is known as tenorite. It is a product of copper mining and the precursor to many other copper-containing products and chemical compounds. Production It is produced on a large scale by pyrometallurgy, as one stage in extracting copper from its ores. The ores are treated with an aqueous mixture of ammonium carbonate, ammonia, and oxygen to give copper(I) and copper(II) ammine complexes, which are extracted from the solids. These complexes are decomposed with steam to give CuO. It can be formed by heating copper in air at around 300–800°C: : 2 Cu + O2 → 2 CuO For laboratory uses, pure copper(II) oxide is better prepared by heating copper(II) nitrate, copper(II) hydroxide, or basic copper(II) carbonate: : 2 Cu(NO3)2(s) → 2 CuO(s) + 4 NO2(g) + O2(g) (180° ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Tetrafluoroboric Acid
Fluoroboric acid or tetrafluoroboric acid (archaically, fluoboric acid) is an inorganic compound with the chemical formula +BF4−], where H+ represents the solvated proton. The solvent can be any suitably Lewis-basic entity. For instance, in water, it can be represented by (oxonium tetrafluoroborate), although more realistically, several water molecules solvate the proton: (H2O)''n''+BF4−]. The ethyl ether solvate is also commercially available: (Et2O)''n''+BF4−], where ''n'' is most likely 2. Unlike other strong acids like H2SO4 or HClO4, the pure unsolvated substance does not exist (see below). It is mainly produced as a precursor to other fluoroborate salts.Gregory K. Friestad, Bruce P. Branchaud "Tetrafluoroboric Acid" E-Eros Encyclopedia of Reagents for Organic Synthesis. It is a strong acid. Fluoroboric acid is corrosive and attacks the skin. It is available commercially as a solution in water and other solvents such as diethyl ether. It is a strong acid with a ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Copper
Copper is a chemical element with the symbol Cu (from la, cuprum) and atomic number 29. It is a soft, malleable, and ductile metal with very high thermal and electrical conductivity. A freshly exposed surface of pure copper has a pinkish-orange color. Copper is used as a conductor of heat and electricity, as a building material, and as a constituent of various metal alloys, such as sterling silver used in jewelry, cupronickel used to make marine hardware and coins, and constantan used in strain gauges and thermocouples for temperature measurement. Copper is one of the few metals that can occur in nature in a directly usable metallic form ( native metals). This led to very early human use in several regions, from circa 8000 BC. Thousands of years later, it was the first metal to be smelted from sulfide ores, circa 5000 BC; the first metal to be cast into a shape in a mold, c. 4000 BC; and the first metal to be purposely alloyed with another metal, tin, to create ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |


_ion_in_aqueous_solution.jpg)