Copper(II) Chloride on:
[Wikipedia]
[Google]
[Amazon]
Copper(II) chloride is the
Standard X-ray Diffraction Powder Patterns
National Bureau of Standards, Monograph 25, Section 18; page 33.
 Aqueous solution prepared from copper(II) chloride contain a range of copper(II) complexes depending on concentration, temperature, and the presence of additional chloride ions. These species include blue color of u(H2O)6sup>2+ and yellow or red color of the halide complexes of the formula uCl2+xsup>x−.
Aqueous solution prepared from copper(II) chloride contain a range of copper(II) complexes depending on concentration, temperature, and the presence of additional chloride ions. These species include blue color of u(H2O)6sup>2+ and yellow or red color of the halide complexes of the formula uCl2+xsup>x−.
 Partial hydrolysis gives dicopper chloride trihydroxide, Cu2(OH)3Cl, a popular fungicide.
Partial hydrolysis gives dicopper chloride trihydroxide, Cu2(OH)3Cl, a popular fungicide.
 This reaction is performed in a polar solvent such as
This reaction is performed in a polar solvent such as  Such compounds are intermediates in the synthesis of BINAP and its derivatives.
Copper(II) chloride dihydrate promotes the hydrolysis of
Such compounds are intermediates in the synthesis of BINAP and its derivatives.
Copper(II) chloride dihydrate promotes the hydrolysis of 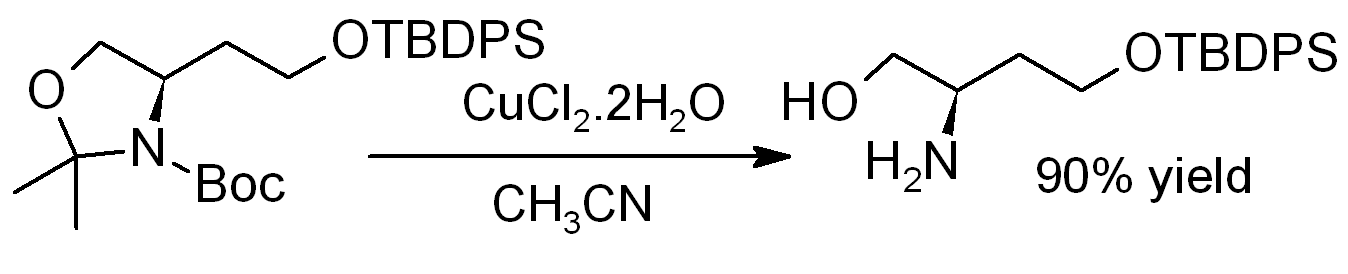 CuCl2 also catalyses the
CuCl2 also catalyses the
Copper Chloride
at '' The Periodic Table of Videos'' (University of Nottingham)
Copper (II) Chloride – Description and Pictures
{{Chlorides Copper(II) compounds Chlorides Metal halides Semiconductor materials Coordination complexes Pyrotechnic colorants
chemical compound
A chemical compound is a chemical substance composed of many identical molecules (or molecular entities) containing atoms from more than one chemical element held together by chemical bonds. A molecule consisting of atoms of only one ele ...
with the chemical formula
In chemistry, a chemical formula is a way of presenting information about the chemical proportions of atoms that constitute a particular chemical compound or molecule, using chemical element symbols, numbers, and sometimes also other symbols, ...
CuCl2. The anhydrous form is yellowish brown but slowly absorbs moisture to form a blue-green dihydrate.
Both the anhydrous and the dihydrate forms occur naturally as the very rare minerals tolbachite and eriochalcite, respectively.Marlene C. Morris, Howard F. McMurdie, Eloise H. Evans, Boris Paretzkin, Harry S. Parker, and Nicolas C. Panagiotopoulos (1981) ''Copper chloride hydrate (eriochalcite)'', in Standard X-ray Diffraction Powder Patterns
National Bureau of Standards, Monograph 25, Section 18; page 33.
Structure
Anhydrous CuCl2 adopts a distortedcadmium iodide
Cadmium iodide is the inorganic compound with the formula CdI2. It is a white hygroscopic solid. It also can be obtained as a mono- and tetrahydrate. It has few applications. It is notable for its crystal structure, which is typical for compound ...
structure. In this motif, the copper centers are octahedral. Most copper(II) compounds exhibit distortions from idealized octahedral geometry due to the Jahn-Teller effect, which in this case describes the localization of one d-electron into a molecular orbital
In chemistry, a molecular orbital is a mathematical function describing the location and wave-like behavior of an electron in a molecule. This function can be used to calculate chemical and physical properties such as the probability of findi ...
that is strongly antibonding with respect to a pair of chloride ligands. In CuCl2·2H2O, the copper again adopts a highly distorted octahedral geometry, the Cu(II) centers being surrounded by two water ligands and four chloride ligands, which bridge
A bridge is a structure built to span a physical obstacle (such as a body of water, valley, road, or rail) without blocking the way underneath. It is constructed for the purpose of providing passage over the obstacle, which is usually somethi ...
asymmetrically to other Cu centers.
Copper(II) chloride is paramagnetic
Paramagnetism is a form of magnetism whereby some materials are weakly attracted by an externally applied magnetic field, and form internal, induced magnetic fields in the direction of the applied magnetic field. In contrast with this behavior, ...
. Of historical interest, CuCl2·2H2O was used in the first electron paramagnetic resonance
Electron paramagnetic resonance (EPR) or electron spin resonance (ESR) spectroscopy is a method for studying materials that have unpaired electrons. The basic concepts of EPR are analogous to those of nuclear magnetic resonance (NMR), but the sp ...
measurements by Yevgeny Zavoisky
Yevgeny Konstantinovich Zavoisky (russian: Евгений Константинович Завойский; September 28, 1907 – October 9, 1976) was a Soviet physicist known for discovery of electron paramagnetic resonance in 1944. He likely obs ...
in 1944.
Properties and reactions
Hydrolysis
Copper(II) hydroxide precipitates upon treating copper(II) chloride solutions with base: :CuCl2 + 2 NaOH → Cu(OH)2 + 2 NaCl Partial hydrolysis gives dicopper chloride trihydroxide, Cu2(OH)3Cl, a popular fungicide.
Partial hydrolysis gives dicopper chloride trihydroxide, Cu2(OH)3Cl, a popular fungicide.
Redox
Copper(II) chloride is a mild oxidant. It decomposes to copper(I) chloride andchlorine gas
Chlorine is a chemical element with the symbol Cl and atomic number 17. The second-lightest of the halogens, it appears between fluorine and bromine in the periodic table and its properties are mostly intermediate between them. Chlorine is ...
near 1000 °C:
:2 CuCl2 → 2 CuCl + Cl2
Copper(II) chloride (CuCl2) reacts with several metals to produce copper metal or copper(I) chloride (CuCl) with oxidation of the other metal. To convert copper(II) chloride to copper(I) chloride, it can be convenient to reduce an aqueous solution with sulfur dioxide
Sulfur dioxide ( IUPAC-recommended spelling) or sulphur dioxide (traditional Commonwealth English) is the chemical compound with the formula . It is a toxic gas responsible for the odor of burnt matches. It is released naturally by volcanic ...
as the reductant:
:2 CuCl2 + SO2 + 2 H2O → 2 CuCl + 2 HCl + H2SO4
Coordination complexes
CuCl2 reacts with HCl or otherchloride
The chloride ion is the anion (negatively charged ion) Cl−. It is formed when the element chlorine (a halogen) gains an electron or when a compound such as hydrogen chloride is dissolved in water or other polar solvents. Chloride s ...
sources to form complex ions: the red CuCl3− (it is a dimer in reality, Cu2Cl62−, a couple of tetrahedrons that share an edge), and the green or yellow CuCl42−.
: +
: + 2
Some of these complexes can be crystallized from aqueous solution, and they adopt a wide variety of structures.
Copper(II) chloride also forms a variety of coordination complexes
A coordination complex consists of a central atom or ion, which is usually metallic and is called the ''coordination centre'', and a surrounding array of bound molecules or ions, that are in turn known as ''ligands'' or complexing agents. Many ...
with ligand
In coordination chemistry, a ligand is an ion or molecule ( functional group) that binds to a central metal atom to form a coordination complex. The bonding with the metal generally involves formal donation of one or more of the ligand's ele ...
s such as ammonia
Ammonia is an inorganic compound of nitrogen and hydrogen with the formula . A stable binary hydride, and the simplest pnictogen hydride, ammonia is a colourless gas with a distinct pungent smell. Biologically, it is a common nitrogeno ...
, pyridine
Pyridine is a basic (chemistry), basic heterocyclic compound, heterocyclic organic compound with the chemical formula . It is structurally related to benzene, with one methine group replaced by a nitrogen atom. It is a highly flammable, weakl ...
and triphenylphosphine oxide:
:CuCl2 + 2 C5H5N → uCl2(C5H5N)2(tetragonal)
:CuCl2 + 2 (C6H5)3PO → uCl2((C6H5)3PO)2(tetrahedral)
However "soft" ligands such as phosphine
Phosphine ( IUPAC name: phosphane) is a colorless, flammable, highly toxic compound with the chemical formula , classed as a pnictogen hydride. Pure phosphine is odorless, but technical grade samples have a highly unpleasant odor like rotti ...
s (e.g., triphenylphosphine
Triphenylphosphine (IUPAC name: triphenylphosphane) is a common organophosphorus compound with the formula P(C6H5)3 and often abbreviated to P Ph3 or Ph3P. It is widely used in the synthesis of organic and organometallic compounds. PPh3 exists ...
), iodide, and cyanide
Cyanide is a naturally occurring, rapidly acting, toxic chemical that can exist in many different forms.
In chemistry, a cyanide () is a chemical compound that contains a functional group. This group, known as the cyano group, consists of ...
as well as some tertiary amine
In chemistry, amines (, ) are compounds and functional groups that contain a basic nitrogen atom with a lone pair. Amines are formally derivatives of ammonia (), wherein one or more hydrogen atoms have been replaced by a substituent su ...
s induce reduction to give copper(I) complexes.
Preparation
Copper(II) chloride is prepared commercially by the action of chlorination of copper. Copper at red heat (300-400°C) combines directly with chlorine gas, giving (molten) copper (II) chloride. The reaction is very exothermic. : Cu(''s'') + Cl2(''g'') → CuCl2(''l'') It is also commercially practical to combinecopper(II) oxide
Copper(II) oxide or cupric oxide is an inorganic compound with the formula CuO. A black solid, it is one of the two stable oxides of copper, the other being Cu2O or copper(I) oxide (cuprous oxide). As a mineral, it is known as tenorite. It ...
with an excess of ammonium chloride at similar temperatures, producing copper chloride, ammonia, and water:
: CuO + 2NH4Cl → CuCl2 + 2NH3 + H2O
Although copper metal itself cannot be oxidised by hydrochloric acid
Hydrochloric acid, also known as muriatic acid, is an aqueous solution of hydrogen chloride. It is a colorless solution with a distinctive pungent smell. It is classified as a strong acid. It is a component of the gastric acid in the dig ...
, copper-containing bases such as the hydroxide, oxide, or copper(II) carbonate
Copper(II) carbonate or cupric carbonate is a chemical compound with formula . At ambient temperatures, it is an ionic solid (a salt) consisting of copper(II) cations and carbonate anions .
This compound is rarely encountered because it is diff ...
can react to form CuCl2 in an acid-base reaction.
Once prepared, a solution of CuCl2 may be purified by crystallization
Crystallization is the process by which solid forms, where the atoms or molecules are highly organized into a structure known as a crystal. Some ways by which crystals form are precipitating from a solution, freezing, or more rarely de ...
. A standard method takes the solution mixed in hot dilute hydrochloric acid
Hydrochloric acid, also known as muriatic acid, is an aqueous solution of hydrogen chloride. It is a colorless solution with a distinctive pungent smell. It is classified as a strong acid. It is a component of the gastric acid in the dig ...
, and causes the crystals to form by cooling in a Calcium chloride (CaCl2)-ice bath.S. H. Bertz, E. H. Fairchild, in ''Handbook of Reagents for Organic Synthesis, Volume 1: Reagents, Auxiliaries and Catalysts for C-C Bond Formation'', (R. M. Coates, S. E. Denmark, eds.), pp. 220-3, Wiley, New York, 1738.
There are indirect and rarely used means of using copper ions in solution to form copper(II) chloride. Electrolysis of aqueous sodium chloride with copper electrodes produces (among other things) a blue-green foam that can be collected and converted to the hydrate. While this is not usually done due to the emission of toxic chlorine gas, and the prevalence of the more general chloralkali process
The chloralkali process (also chlor-alkali and chlor alkali) is an industrial process for the electrolysis of sodium chloride (NaCl) solutions. It is the technology used to produce chlorine and sodium hydroxide (caustic soda), which are commodit ...
, the electrolysis will convert the copper metal to copper ions in solution forming the compound. Indeed, any solution of copper ions can be mixed with hydrochloric acid and made into a copper chloride by removing any other ions.
Natural occurrence
Copper(II) chloride occurs naturally as the very rare anhydrous mineral tolbachite and the dihydrate eriochalcite. Both are found nearfumarole
A fumarole (or fumerole) is a vent in the surface of the Earth or other rocky planet from which hot volcanic gases and vapors are emitted, without any accompanying liquids or solids. Fumaroles are characteristic of the late stages of volcan ...
s and in some Cu mines. More common are mixed oxyhydroxide-chlorides like atacamite Cu2(OH)3Cl, arising among Cu ore beds oxidation zones in arid climate (also known from some altered slags).
Uses
In organic synthesis
Co-catalyst in Wacker process
A major industrial application for copper(II) chloride is as a co-catalyst with palladium(II) chloride in theWacker process
The Wacker process or the Hoechst-Wacker process (named after the chemical companies of the same name) refers to the oxidation of ethylene to acetaldehyde in the presence of palladium(II) chloride as the catalyst. This chemical reaction was one of ...
. In this process, ethene (ethylene) is converted to ethanal (acetaldehyde) using water and air. During the reaction, PdCl2 is reduced to Pd, and the CuCl2 serves to re-oxidize this back to PdCl2. Air can then oxidize the resultant CuCl back to CuCl2, completing the cycle.
# C2H4 + PdCl2 + H2O → CH3CHO + Pd + 2 HCl
# Pd + 2 CuCl2 → 2 CuCl + PdCl2
# 4 CuCl + 4 HCl + O2 → 4 CuCl2 + 2 H2O
The overall process is:
:2 C2H4 + O2 → 2 CH3CHO
Other organic synthetic applications
Copper(II) chloride has some highly specialized applications in the synthesis of organic compounds. It affects chlorination of aromatic hydrocarbons—this is often performed in the presence of aluminium oxide. It is able to chlorinate the alpha position ofcarbonyl
In organic chemistry, a carbonyl group is a functional group composed of a carbon atom double-bonded to an oxygen atom: C=O. It is common to several classes of organic compounds, as part of many larger functional groups. A compound containin ...
compounds:
: This reaction is performed in a polar solvent such as
This reaction is performed in a polar solvent such as dimethylformamide
Dimethylformamide is an organic compound with the formula ( CH3)2NC(O)H. Commonly abbreviated as DMF (although this initialism is sometimes used for dimethylfuran, or dimethyl fumarate), this colourless liquid is miscible with water and the maj ...
(DMF), often in the presence of lithium chloride, which accelerates the reaction.
CuCl2, in the presence of oxygen
Oxygen is the chemical element with the symbol O and atomic number 8. It is a member of the chalcogen group in the periodic table, a highly reactive nonmetal, and an oxidizing agent that readily forms oxides with most elements as we ...
, can also oxidize phenols
In organic chemistry, phenols, sometimes called phenolics, are a class of chemical compounds consisting of one or more hydroxyl groups (— O H) bonded directly to an aromatic hydrocarbon group. The simplest is phenol, . Phenolic compounds ...
. The major product can be directed to give either a quinone
The quinones are a class of organic compounds that are formally "derived from aromatic compounds benzene.html" ;"title="uch as benzene">uch as benzene or naphthalene] by conversion of an even number of –CH= groups into –C(=O)– groups with ...
or a coupled product from oxidative dimerization. The latter process provides a high-yield route to 1,1-binaphthol:
: Such compounds are intermediates in the synthesis of BINAP and its derivatives.
Copper(II) chloride dihydrate promotes the hydrolysis of
Such compounds are intermediates in the synthesis of BINAP and its derivatives.
Copper(II) chloride dihydrate promotes the hydrolysis of acetonides
In organic chemistry, an acetonide is the functional group composed of the cyclic ketal of a diol with acetone. The more systematic name for this structure is an isopropylidene ketal. Acetonide is a common protecting group for 1,2- and 1,3- ...
, i.e., for deprotection to regenerate diols or aminoalcohols, as in this example (where TBDPS = ''tert''-butyldiphenylsilyl):
: CuCl2 also catalyses the
CuCl2 also catalyses the free radical
A daughter category of ''Ageing'', this category deals only with the biological aspects of ageing.
Ageing
Ailments of unknown cause
Biogerontology
Biological processes
Causes of death
Cellular processes
Gerontology
Life extension
Metabo ...
addition of sulfonyl chlorides to alkene
In organic chemistry, an alkene is a hydrocarbon containing a carbon–carbon double bond.
Alkene is often used as synonym of olefin, that is, any hydrocarbon containing one or more double bonds.H. Stephen Stoker (2015): General, Organic, an ...
s; the alpha-chlorosulfone may then undergo elimination
Elimination may refer to:
Science and medicine
* Elimination reaction, an organic reaction in which two functional groups split to form an organic product
*Bodily waste elimination, discharging feces, urine, or foreign substances from the bo ...
with base to give a vinyl sulfone product.
In inorganic synthesis
Catalyst in production of chlorine
Copper(II) chloride is used as a catalyst in a variety of processes that produce chlorine by oxychlorination. TheDeacon process
The Deacon process, invented by Henry Deacon, is a process used during the manufacture of alkalis (the initial end product was sodium carbonate) by the Leblanc process. Hydrogen chloride gas was converted to chlorine gas, which was then used to ...
takes place at about 400 to 450 °C in the presence of a copper chloride:
:4 HCl + O2 → 2 Cl2 + 2 H2O
Copper(II) chloride catalyzes the chlorination in the production of vinyl chloride and dichloroethane.H.Wayne Richardson, "Copper Compounds" in Ullmann's Encyclopedia of Industrial Chemistry, 2005, Wiley-VCH, Weinheim,
Copper(II) chloride is used in the Copper–chlorine cycle in which it splits steam into a copper oxygen compound and hydrogen chloride, and is later recovered in the cycle from the electrolysis of copper(I) chloride.
Niche uses
Copper(II) chloride is also used inpyrotechnics
Pyrotechnics is the science and craft of creating such things as fireworks, safety matches, oxygen candles, Pyrotechnic fastener, explosive bolts and other fasteners, parts of automotive airbags, as well as gas-pressure blasting in mining, quarry ...
as a blue/green coloring agent. In a flame test
A flame test is an analytical procedure used in chemistry to detect the presence of certain elements, primarily metal ions, based on each element's characteristic flame emission spectrum (which may be affected by the presence of chloride ...
, copper chlorides, like all copper compounds, emit green-blue.
In humidity indicator cards (HICs), cobalt-free brown to azure (copper(II) chloride base) HICs can be found on the market. In 1998, the European Community
The European Economic Community (EEC) was a regional organization created by the Treaty of Rome of 1957,Today the largely rewritten treaty continues in force as the ''Treaty on the functioning of the European Union'', as renamed by the Lisb ...
(EC) classified items containing cobalt(II) chloride of 0.01 to 1% w/w as T (Toxic), with the corresponding R phrase of R49 (may cause cancer if inhaled). As a consequence, new cobalt-free humidity indicator cards have been developed that contain copper.
Safety
Copper(II) chloride can be toxic. Only concentrations below 5 ppm are allowed in drinking water by the US Environmental Protection Agency.References
Further reading
# # # ''The Merck Index'', 7th edition, Merck & Co, Rahway, New Jersey, USA, 1960. # D. Nicholls, ''Complexes and First-Row Transition Elements'', Macmillan Press, London, 1973. # A. F. Wells, Structural Inorganic Chemistry'', 5th ed., Oxford University Press, Oxford, UK, 1984. # J. March, ''Advanced Organic Chemistry'', 4th ed., p. 723, Wiley, New York, 1992. # ''Fieser & Fieser Reagents for Organic Synthesis'' Volume 5, p158, Wiley, New York, 1975. #External links
Copper Chloride
at '' The Periodic Table of Videos'' (University of Nottingham)
Copper (II) Chloride – Description and Pictures
{{Chlorides Copper(II) compounds Chlorides Metal halides Semiconductor materials Coordination complexes Pyrotechnic colorants