|
Cobalt Tris(diethyldithiocarbamate)
Cobalt tris(diethyldithiocarbamate) is the coordination complex of cobalt with diethyldithiocarbamate with the formula Co(S2CNEt2)3 (Et = ethyl). It is a diamagnetic green solid that is soluble in organic solvents. Synthesis, structure, bonding Cobalt tris(dithiocarbamate)s are typically are prepared by air-oxidation of mixtures of dithiocarbamate salts and a cobalt(II) nitrate. Cobalt tris(diethyldithiocarbamate) is an octahedral coordination complex of low-spin Co(III) with idealized D3 symmetry. The Co-S distances are 267 pm. Reactions Oxidation of Co(Et2dtc)3 occurs at mild potentials to give the cobalt(IV) derivative. Treatment of Co(Et2dtc)3 with fluoroboric acid Fluoroboric acid or tetrafluoroboric acid (archaically, fluoboric acid) is an inorganic compound with the chemical formula +BF4−], where H+ represents the solvated proton. The solvent can be any suitably Lewis-basic entity. For instance, in w ... results in the removal of 0.5 equiv of ligand giving a binucle ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Transition Metal Dithiocarbamate Complexes
133px, Tris(dithiocarbamate) complexes have idealized D3 symmetry. Transition metal dithiocarbamate complexes are coordination complexes containing one or more dithiocarbamate ligand, which are typically abbreviated R2dtc−. Many complexes are known. Several homoleptic derivatives have the formula M(R2dtc)n where n = 2 and 3. Ligand characteristics Dithiocarbamates are anions. Because of the pi-donor properties of the amino substituent, the two sulfur centers show enhanced basicity. This situation is represented by the zwitterionic resonance structure that depicts a positive charge on N and negative charges on both sulfurs. This N to C pi-bonding results in partial double bond character. Consequently, barriers to rotational about this bond are elevated. Another consequence of their high basicity, dithiocarbamates often stabilize complexes in uncharacteristically high oxidation state (e.g., Fe(IV), Co(IV), Ni(III), Cu(III)). Dithiocarbamate salts are easily synthesized. ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Diethyldithiocarbamate
Sodium diethyldithiocarbamate is the organosulfur compound with the formula NaS2CN(C2H5)2. It is a pale yellow, water soluble salt. Preparation This salt is obtained by treating carbon disulfide with diethylamine in the presence of sodium hydroxide: :CS2 + HN(C2H5)2 + NaOH → NaS2CN(C2H5)2 + H2O Other dithiocarbamates can be prepared similarly from secondary amines and carbon disulfide. They are used as chelating agents for transition metal ions and as precursors to herbicides and vulcanization reagents. Oxidation to thiuram disulfide Oxidation of sodium diethyldithiocarbamate gives the disulfide, also called a thiuram disulfide (Et = ethyl): :2 NaS2CNEt2 + I2 → Et2NC(S)S-SC(S)NEt2 + 2 NaI Ligand bonding The diethyldithiocarbamate anion forms the basis of transition metal dithiocarbamate complexes. The ligands coordinate to many " softer" metals via the two sulfur atoms. Other more complicated bonding modes are known including binding as unidentate liga ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Ethyl Group
In organic chemistry, an ethyl group (abbr. Et) is an alkyl substituent with the formula , derived from ethane (). ''Ethyl'' is used in the International Union of Pure and Applied Chemistry's nomenclature of organic chemistry for a saturated two-carbon moiety in a molecule, while the prefix "''eth-''" is used to indicate the presence of two carbon atoms in the molecule. Ethylation Ethylation is the formation of a compound by introduction of the ethyl group. The most widely practiced example of this reaction is the ethylation of benzene with ethylene to yield ethylbenzene, a precursor to styrene, which is a precursor to polystyrene. Approximately 24.7 million tons of ethylbenzene were produced in 1999. :: Many ethyl-containing compounds are generated by electrophilic ethylation, i.e. treatment of nucleophiles with sources of Et+. Triethyloxonium tetrafluoroborate t3OF4 is such a reagent. For good nucleophiles, less electrophilic reagents are employed, such as ethyl h ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Coordination Complex
A coordination complex consists of a central atom or ion, which is usually metallic and is called the ''coordination centre'', and a surrounding array of bound molecules or ions, that are in turn known as ''ligands'' or complexing agents. Many metal-containing compounds, especially those that include transition metals (elements like titanium that belong to the Periodic Table's d-block), are coordination complexes. Nomenclature and terminology Coordination complexes are so pervasive that their structures and reactions are described in many ways, sometimes confusingly. The atom within a ligand that is bonded to the central metal atom or ion is called the donor atom. In a typical complex, a metal ion is bonded to several donor atoms, which can be the same or different. A polydentate (multiple bonded) ligand is a molecule or ion that bonds to the central atom through several of the ligand's atoms; ligands with 2, 3, 4 or even 6 bonds to the central atom are common. These compl ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Symmetry Group
In group theory, the symmetry group of a geometric object is the group of all transformations under which the object is invariant, endowed with the group operation of composition. Such a transformation is an invertible mapping of the ambient space which takes the object to itself, and which preserves all the relevant structure of the object. A frequent notation for the symmetry group of an object ''X'' is ''G'' = Sym(''X''). For an object in a metric space, its symmetries form a subgroup of the isometry group of the ambient space. This article mainly considers symmetry groups in Euclidean geometry, but the concept may also be studied for more general types of geometric structure. Introduction We consider the "objects" possessing symmetry to be geometric figures, images, and patterns, such as a wallpaper pattern. For symmetry of physical objects, one may also take their physical composition as part of the pattern. (A pattern may be specified formally as a scalar field, a funct ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
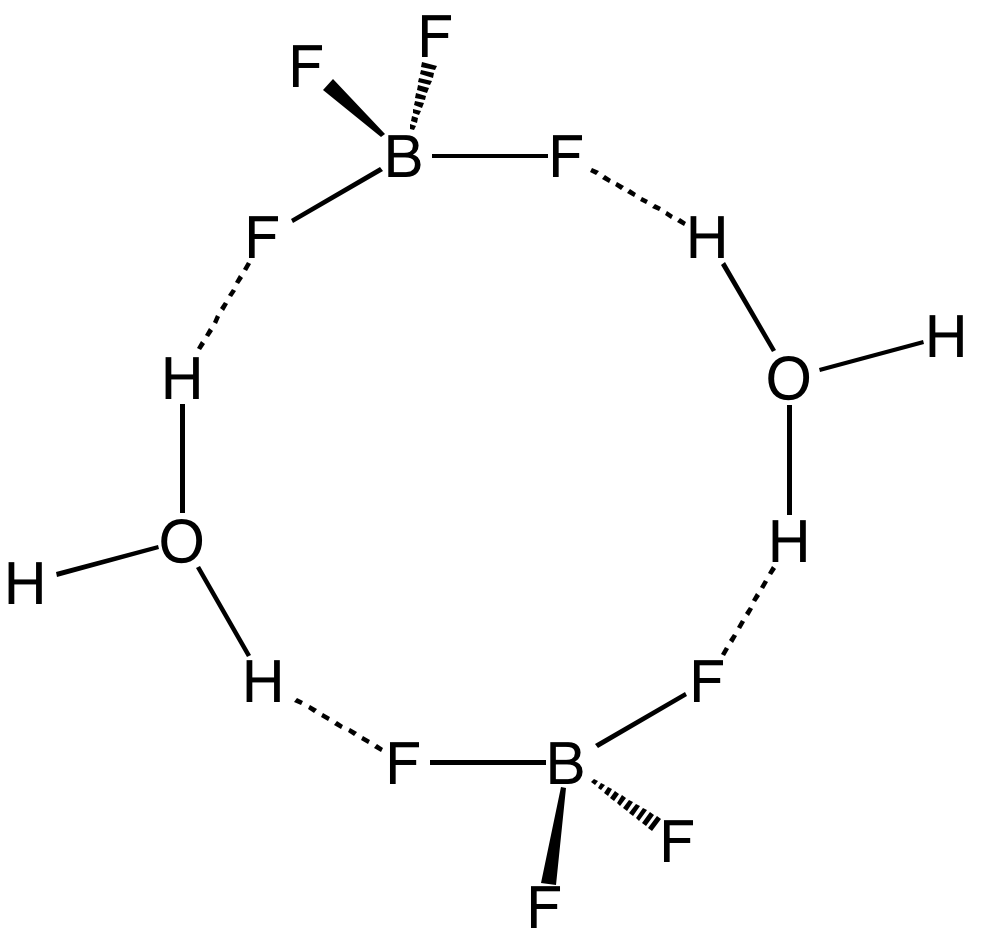
Fluoroboric Acid
Fluoroboric acid or tetrafluoroboric acid (archaically, fluoboric acid) is an inorganic compound with the chemical formula +BF4−], where H+ represents the solvated proton. The solvent can be any suitably Lewis-basic entity. For instance, in water, it can be represented by (oxonium tetrafluoroborate), although more realistically, several water molecules solvate the proton: (H2O)''n''+BF4−]. The ethyl ether solvate is also commercially available: (Et2O)''n''+BF4−], where ''n'' is most likely 2. Unlike other strong acids like H2SO4 or HClO4, the pure unsolvated substance does not exist (see below). It is mainly produced as a precursor to other fluoroborate salts.Gregory K. Friestad, Bruce P. Branchaud "Tetrafluoroboric Acid" E-Eros Encyclopedia of Reagents for Organic Synthesis. It is a strong acid. Fluoroboric acid is corrosive and attacks the skin. It is available commercially as a solution in water and other solvents such as diethyl ether. It is a strong acid with a ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Iron Tris(diethyldithiocarbamate)
Iron tris(diethyldithiocarbamate) is the coordination complex of iron with diethyldithiocarbamate with the formula Fe(S2CNEt2)3 (Et = ethyl). It is a black solid that is soluble in organic solvents. Synthesis, structure, bonding Iron tris(dithiocarbamate)s are typically are prepared by salt metathesis reactions. Iron tris(diethyldithiocarbamate) is an octahedral coordination complex of iron(III) with idealized D3 symmetry. The phenomenon of spin crossover (SCO) was first reported in 1931 by Cambi ''et al.'' who observed anomalous magnetic behavior for the tris(N,N-dialkyldithiocarbamatoiron(III) complexes. The magnetism of these complexes are sensitive to the nature of the amine substituents as well as to temperature. This behavior is consistent with an equilibrium between two or more spin states. In the case of Fe(Et2dtc)3, X-ray crystallography reveals that the Fe-S bonds are 231 pm at 79K but 356 pm at 297 K. These data indicate a low-spin configuration at low temperatures an ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Dithiocarbamates
In organic chemistry, a dithiocarbamate is a functional group with the general formula and structure . It is the analog of a carbamate in which both oxygen atoms are replaced by sulfur atoms (when only 1 oxygen is replaced the result is thiocarbamate). A common example is sodium diethyldithiocarbamate. Dithiocarbamates and their derivatives are widely used in the vulcanization of rubber. Formation Many primary and secondary amines react with carbon disulfide and sodium hydroxide to form dithiocarbamate salts: :R2NH + CS2 + NaOH → R2NCS2−Na+ + H2O Ammonia reacts with CS2 similarly: :2 NH3 + CS2 → H2NCS2−NH4+ Dithiocarbamate salts are pale colored solids that are soluble in water and polar organic solvents. Reactions Dithiocarbamates are readily S-alkylated. Thus, methyl dimethyldithiocarbamate can be prepared by methylation of the dithiocarbamate: :(CH3)2NCS2Na + (CH3O)2SO2 → (CH3)2NC(S)SCH3 + Na H3OSO3 Oxidation of dithiocarbamates gives the ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |