Fluoroboric acid on:
[Wikipedia]
[Google]
[Amazon]
Fluoroboric acid or tetrafluoroboric acid (archaically, fluoboric acid) is an
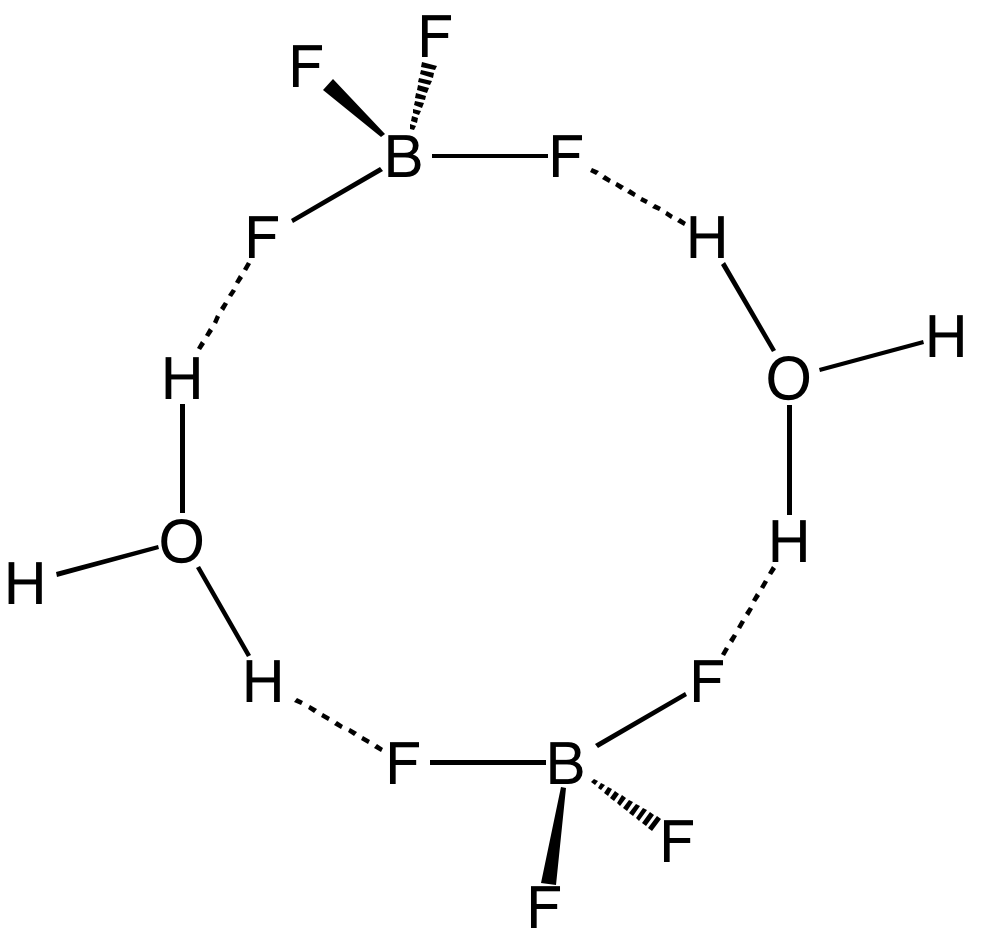 Aqueous solutions of HBF4 are produced by dissolving
Aqueous solutions of HBF4 are produced by dissolving
inorganic compound
In chemistry, an inorganic compound is typically a chemical compound that lacks carbon–hydrogen bonds, that is, a compound that is not an organic compound. The study of inorganic compounds is a subfield of chemistry known as '' inorganic chemi ...
with the chemical formula
In chemistry, a chemical formula is a way of presenting information about the chemical proportions of atoms that constitute a particular chemical compound or molecule, using chemical element symbols, numbers, and sometimes also other symbols, ...
+BF4−], where H+ represents the solvated proton. The solvent can be any suitably Lewis-basic entity. For instance, in water, it can be represented by (oxonium tetrafluoroborate), although more realistically, several water molecules solvate the proton: (H2O)''n''+BF4−]. The ethyl ether solvate is also commercially available: (Et2O)''n''+BF4−], where ''n'' is most likely 2. Unlike other strong acids like H2SO4 or HClO4, the pure unsolvated substance does not exist (see below).
It is mainly produced as a precursor to other fluoroborate salts.Gregory K. Friestad, Bruce P. Branchaud "Tetrafluoroboric Acid" E-Eros Encyclopedia of Reagents for Organic Synthesis. It is a strong acid. Fluoroboric acid is corrosive and attacks the skin. It is available commercially as a solution in water and other solvent
A solvent (s) (from the Latin '' solvō'', "loosen, untie, solve") is a substance that dissolves a solute, resulting in a solution. A solvent is usually a liquid but can also be a solid, a gas, or a supercritical fluid. Water is a solvent for ...
s such as diethyl ether
Diethyl ether, or simply ether, is an organic compound in the ether class with the formula , sometimes abbreviated as (see Pseudoelement symbols). It is a colourless, highly volatile, sweet-smelling ("ethereal odour"), extremely flammable liq ...
. It is a strong acid with a weakly coordinating, non-oxidizing conjugate base. It is structurally similar to perchloric acid
Perchloric acid is a mineral acid with the formula H Cl O4. Usually found as an aqueous solution, this colorless compound is a stronger acid than sulfuric acid, nitric acid and hydrochloric acid. It is a powerful oxidizer when hot, but aqueous s ...
, but lacks the hazards associated with oxidant
An oxidizing agent (also known as an oxidant, oxidizer, electron recipient, or electron acceptor) is a substance in a redox chemical reaction that gains or "Electron acceptor, accepts"/"receives" an electron from a (called the , , or ). In ...
s.
Structure and production
Pure HBF4 has been described as a "nonexistent compound", as a sufficiently 'naked' proton is expected to abstract a fluoride from the tetrafluoroborate ion to give hydrogen fluoride andboron trifluoride
Boron trifluoride is the inorganic compound with the formula BF3. This pungent, colourless, and toxic gas forms white fumes in moist air. It is a useful Lewis acid and a versatile building block for other boron compounds.
Structure and bondin ...
: +BF4–] → HF + BF3. (The same holds true for the superacids that are known by the simple formulas HPF6 and HSbF6.) However, a solution of BF3 in HF is highly acidic, having an approximate speciation of 2F+BF4–] and a Hammett acidity function of –16.6 at 7 mol % BF3, easily qualifying as a superacid. Although the solvent-free HBF4 has not been isolated, its solvates are well characterized. These salts consist of protonated solvent as a cation, e.g., H3O+ and , and the tetrahedral anion. The anion and cations are strongly hydrogen-bonded.
 Aqueous solutions of HBF4 are produced by dissolving
Aqueous solutions of HBF4 are produced by dissolving boric acid
Boric acid, more specifically orthoboric acid, is a compound of boron, oxygen, and hydrogen with formula . It may also be called hydrogen borate or boracic acid. It is usually encountered as colorless crystals or a white powder, that dissolve ...
in aqueous hydrofluoric acid
Hydrofluoric acid is a solution of hydrogen fluoride (HF) in water. Solutions of HF are colourless, acidic and highly corrosive. It is used to make most fluorine-containing compounds; examples include the commonly used pharmaceutical antidepr ...
. Three equivalents of HF react to give the intermediate boron trifluoride and the fourth gives fluoroboric acid:
: B(OH)3 + 4 HF → H3O+ + + 2 H2O
Anhydrous solutions can be prepared by treatment of aqueous fluoroboric acid with acetic anhydride
Acetic anhydride, or ethanoic anhydride, is the chemical compound with the formula (CH3CO)2O. Commonly abbreviated Ac2O, it is the simplest isolable anhydride of a carboxylic acid and is widely used as a reagent in organic synthesis. It is a c ...
.
Acidity
The acidity of fluoroboric acid is complicated by the fact that the name refers to several different species H(OEt2)+BF, H3O+BF, and HF.BF3 – each with a different acidity. The aqueous pKa is quoted as −0.44. Titration of NBuBF in acetonitrile solution indicates that HBF4, i.e., HF.BF3, has a pKa of 1.6 in that solvent. Its acidity is thus comparable to that offluorosulfonic acid
Fluorosulfuric acid (IUPAC name: sulfurofluoridic acid) is the inorganic compound with the chemical formula HSO3F. It is one of the strongest acids commercially available. It is a tetrahedral molecule and is closely related to sulfuric acid, H2SO ...
.
Applications
Fluoroboric acid is the principal precursor to fluoroborate salts, which are typically prepared by treating the metal oxides with fluoroboric acid. The inorganic salts are intermediates in the manufacture of flame-retardant materials and glazingfrit
A frit is a ceramic composition that has been fused, quenched, and granulated. Frits form an important part of the batches used in compounding enamels and ceramic glazes; the purpose of this pre-fusion is to render any soluble and/or toxic com ...
s, and in electrolytic generation of boron. HBF4 is also used in aluminum etching and acid pickling.
Organic chemistry
HBF4 is used as acatalyst
Catalysis () is the process of increasing the rate of a chemical reaction by adding a substance known as a catalyst (). Catalysts are not consumed in the reaction and remain unchanged after it. If the reaction is rapid and the catalyst recyc ...
for alkylation
Alkylation is the transfer of an alkyl group from one molecule to another. The alkyl group may be transferred as an alkyl carbocation, a free radical, a carbanion, or a carbene (or their equivalents). Alkylating agents are reagents for effectin ...
s and polymerizations. In carbohydrate protection reactions, ethereal fluoroboric acid is an efficient and cost-effective catalyst for transacetalation and isopropylidenation reactions. Acetonitrile solutions cleave acetals and some ethers
In organic chemistry, ethers are a class of compounds that contain an ether group—an oxygen atom connected to two alkyl or aryl groups. They have the general formula , where R and R′ represent the alkyl or aryl groups. Ethers can again be ...
. Many reactive cations have been obtained using fluoroboric acid, e.g. tropylium tetrafluoroborate (), triphenylmethyl tetrafluoroborate (), triethyloxonium tetrafluoroborate (), and benzenediazonium tetrafluoroborate ().
Electroplating
Solutions of HBF4 are used in the electroplating of tin and tin alloys. In this application,methanesulfonic acid
Methanesulfonic acid (MsOH) or methanesulphonic acid (in British English) is an organosulfuric, colorless liquid with the chemical formula and structure . It is the simplest of the alkylsulfonic acids (). Salts and esters of methanesulfonic ac ...
is displacing the use of HBF4. Fluoroboric acid is also used for high-speed electroplating of copper in fluoroborate baths.
Safety
HBF4 is toxic and attacks skin and eyes. It attacks glass. It hydrolyzes, releasing corrosive, volatile hydrogen fluoride.Other fluoroboric acids
A series of fluoroboric acids is known in aqueous solutions. The series can be presented as follows: * H (OH)4* H F(OH)3* H F2(OH)2* H F3(OH)* H F4See also
*Fluorosulfuric acid
Fluorosulfuric acid (IUPAC name: sulfurofluoridic acid) is the inorganic compound with the chemical formula HSO3F. It is one of the strongest acids commercially available. It is a tetrahedral molecule and is closely related to sulfuric acid, H2SO4 ...
* Fluoroantimonic acid
Fluoroantimonic acid is a mixture of hydrogen fluoride and antimony pentafluoride, containing various cations and anions (the simplest being and ). This substance is a superacid that can be over a billion times stronger than 100% pure sulfuric ac ...
References
Further reading
* * * * * * * *External links
* {{fluorine compounds Tetrafluoroborates Mineral acids