|
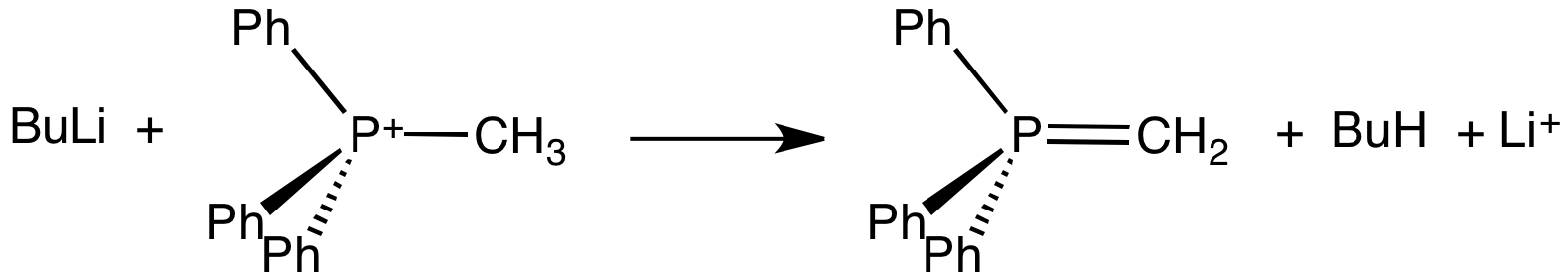
Sulfonium Compounds
In organic chemistry, a sulfonium ion, also known as sulphonium ion or sulfanium ion, is a positively-charged ion (a "cation") featuring three organic substituents attached to sulfur. These organosulfur compounds have the formula . Together with a negatively-charged counterion, they give sulfonium salts. They are typically colorless solids that are soluble in polar organic solvent. Synthesis Sulfonium compounds are usually synthesized by the reaction of thioethers with alkyl halides. For example, the reaction of dimethyl sulfide with iodomethane yields trimethylsulfonium iodide: : The reaction proceeds by a nucleophilic substitution mechanism (SN2), where iodide is the leaving group. For weakly electrophilic alkyl halides, the reactions can be accelerated by the addition of silver tetrafluoroborate. In that vein, the rate (and irreversibility) of methylation improves with more electrophilic methylating agents such as methyl trifluoromethanesulfonate. These S-alkylations ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Methyl Trifluoromethanesulfonate
Methyl trifluoromethanesulfonate, also commonly called methyl triflate and abbreviated MeOTf, is the organic compound with the formula . It is a colourless liquid which finds use in organic chemistry as a powerful methylating agent. The compound is closely related to methyl fluorosulfonate (). Although there has yet to be a reported human fatality, several cases were reported for methyl fluorosulfonate (LC50 (rat, 1 h) = 5 ppm), and methyl triflate is expected to have similar toxicity based on available evidence. Synthesis Methyl triflate is commercially available, however it may also be prepared in the laboratory by treating dimethyl sulfate with triflic acid. : Reactivity Hydrolysis Upon contact with water, methyl triflate loses its methyl group, forming triflic acid and methanol: : Methylation One ranking of methylating agents is . Methyl triflate will alkylate many functional groups which are very poor nucleophiles such as aldehydes, amides, and nitriles. It does not ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Triphenylsulfonium Triflate
Photoacids are molecules that become more acidic upon absorption of light. Either the light causes a photodissociation to produce a strong acid, or the light causes photoassociation (such as a ring forming reaction) that leads to an increased acidity and dissociation of a proton. There are two main types of molecules that release protons upon illumination: photoacid generators (PAGs) and photoacids (PAHs). PAGs undergo proton photodissociation irreversibly, while PAHs are molecules that undergo proton photodissociation and thermal reassociation. In this latter case, the excited state is strongly acidic, but reversible. Photoacid generators An example due to photodissociation is triphenylsulfonium triflate. This colourless salt consists of a sulfonium cation and the triflate anion. Many related salts are known including those with other noncoordinating anions and those with diverse substituents on the phenyl rings. The triphenylsulfonium salts absorb at a wavelength of 233&nb ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
TASF Reagent
The TASF reagent or tris(dimethylamino)sulfonium difluorotrimethylsilicate is a reagent in organic chemistry with structural formula (CH3)2N)3Ssup>+ 2Si(CH3)3sup>−. It is an anhydrous source of fluoride and is used to cleave silyl ether protective groups. Many other fluoride reagents are known, but few are truly anhydrous, because of the extraordinary basicity of "naked" F−. In TASF, the fluoride is masked as an adduct with the weak Lewis acid trimethylsilylfluoride (FSi(CH3)3). The sulfonium cation ((CH3)2N)3S+ is unusually non-electrophilic due to the electron-donating properties of the three (CH3)2N substituents. This compound is prepared from sulfur tetrafluoride: :3 (CH3)2NSi(CH3)3 + SF4 → 2 (CH3)3SiF + (CH3)2N)3Ssup>+ 2Si(CH3)3sup>− The colorless salt precipitates from the reaction solvent, diethyl ether. Structure The cation (CH3)2N)3Ssup>+ is a sulfonium ion. The S-N distances are 1.612 and 1.675 pm. The N-S-N angles are 99.6°. The anion is 2Si(CH ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Johnson–Corey–Chaykovsky Reaction
The Johnson–Corey–Chaykovsky reaction (sometimes referred to as the Corey–Chaykovsky reaction or CCR) is a chemical reaction used in organic chemistry for the synthesis of epoxides, aziridines, and cyclopropanes. It was discovered in 1961 by A. William Johnson and developed significantly by E. J. Corey and Michael Chaykovsky. The reaction involves addition of a sulfur ylide to a ketone, aldehyde, imine, or enone to produce the corresponding 3-membered ring. The reaction is diastereoselective favoring ''trans'' substitution in the product regardless of the initial stereochemistry. The synthesis of epoxides via this method serves as an important retrosynthetic alternative to the traditional epoxidation reactions of olefins. The reaction is most often employed for epoxidation via methylene transfer, and to this end has been used in several notable total syntheses (See Synthesis of epoxides below). Additionally detailed below are the history, mechanism, scope, and enanti ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Ylide
An ylide () or ylid () is a neutral dipolar molecule containing a formally negatively charged atom (usually a carbanion) directly attached to a heteroatom with a formal positive charge (usually nitrogen, phosphorus or sulfur), and in which both atoms have full octets of electrons. The result can be viewed as a structure in which two adjacent atoms are connected by both a covalent and an ionic bond; normally written X+–Y−. Ylides are thus 1,2- dipolar compounds, and a subclass of zwitterions. They appear in organic chemistry as reagents or reactive intermediates. The class name "ylide" for the compound should not be confused with the suffix "-ylide". Resonance structures Many ylides may be depicted by a multiply bonded form in a resonance structure, known as the ylene form, while the actual structure lies in between both forms: : The actual bonding picture of these types of ylides is strictly zwitterionic (the structure on the right) with the strong Coulombic attractio ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Dimethylsulfoniopropionate
Dimethylsulfoniopropionate (DMSP), is an organosulfur compound with the formula (CH3)2S+CH2CH2COO−. This zwitterionic metabolite can be found in marine phytoplankton, seaweeds, and some species of terrestrial and aquatic vascular plants. It functions as an osmolyte as well as several other physiological and environmental roles have also been identified. DMSP was first identified in the marine red alga '' Polysiphonia fastigiata''. Biosynthesis In higher plants, DMSP is biosynthesized from ''S''-methylmethionine. Two intermediates in this conversion are dimethylsulfoniumpropylamine and dimethylsulfoniumpropionaldehyde. In algae, however, the biosynthesis starts with the replacement of the amino group in methionine by hydroxide. Degradation DMSP is broken down by marine microbes to form two major volatile sulfur products, each with distinct effects on the environment. One of its breakdown products is methanethiol (CH3SH), which is assimilated by bacteria into protein sul ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
S-Methylmethionine
''S''-Methylmethionine (SMM) is a derivative of methionine with the chemical formula (Methyl, CH3)2S+CH2CH2CH(NH3+)CO2−. This cation is a naturally-occurring intermediate in many biosynthetic pathways owing to the sulfonium functional group. It is biosynthesized from L-methionine and S-adenosylmethionine, ''S''-adenosylmethionine by the enzyme methionine S-methyltransferase, methionine ''S''-methyltransferase. ''S''-methylmethionine is particularly abundant in plants, being more abundant than methionine. ''S''-Methylmethionine is sometimes referred to as ''vitamin U'', but it is not considered a true vitamin. The term was coined in 1950 by Garnett Cheney for uncharacterized anti-ulcerogenic factors in raw cabbage juice that may help speed healing of peptic ulcers. Biosynthesis and biochemical function ''S''-Methylmethionine arises via the methylation of methionine by S-Adenosyl methionine, ''S''-adenosyl methionine (SAM). The coproduct is S-Adenosyl-L-homocysteine, ''S''-aden ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Biosynthesis
Biosynthesis, i.e., chemical synthesis occurring in biological contexts, is a term most often referring to multi-step, enzyme-Catalysis, catalyzed processes where chemical substances absorbed as nutrients (or previously converted through biosynthesis) serve as enzyme substrate (chemistry), substrates, with conversion by the living organism either into simpler or more complex Product (chemistry), products. Examples of biosynthetic pathways include those for the production of amino acids, lipid membrane components, and nucleotides, but also for the production of all classes of biological macromolecules, and of acetyl-coenzyme A, adenosine triphosphate, nicotinamide adenine dinucleotide and other key intermediate and transactional molecules needed for metabolism. Thus, in biosynthesis, any of an array of Chemical compound, compounds, from simple to complex, are converted into other compounds, and so it includes both the catabolism and anabolism (building up and breaking down) of comple ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
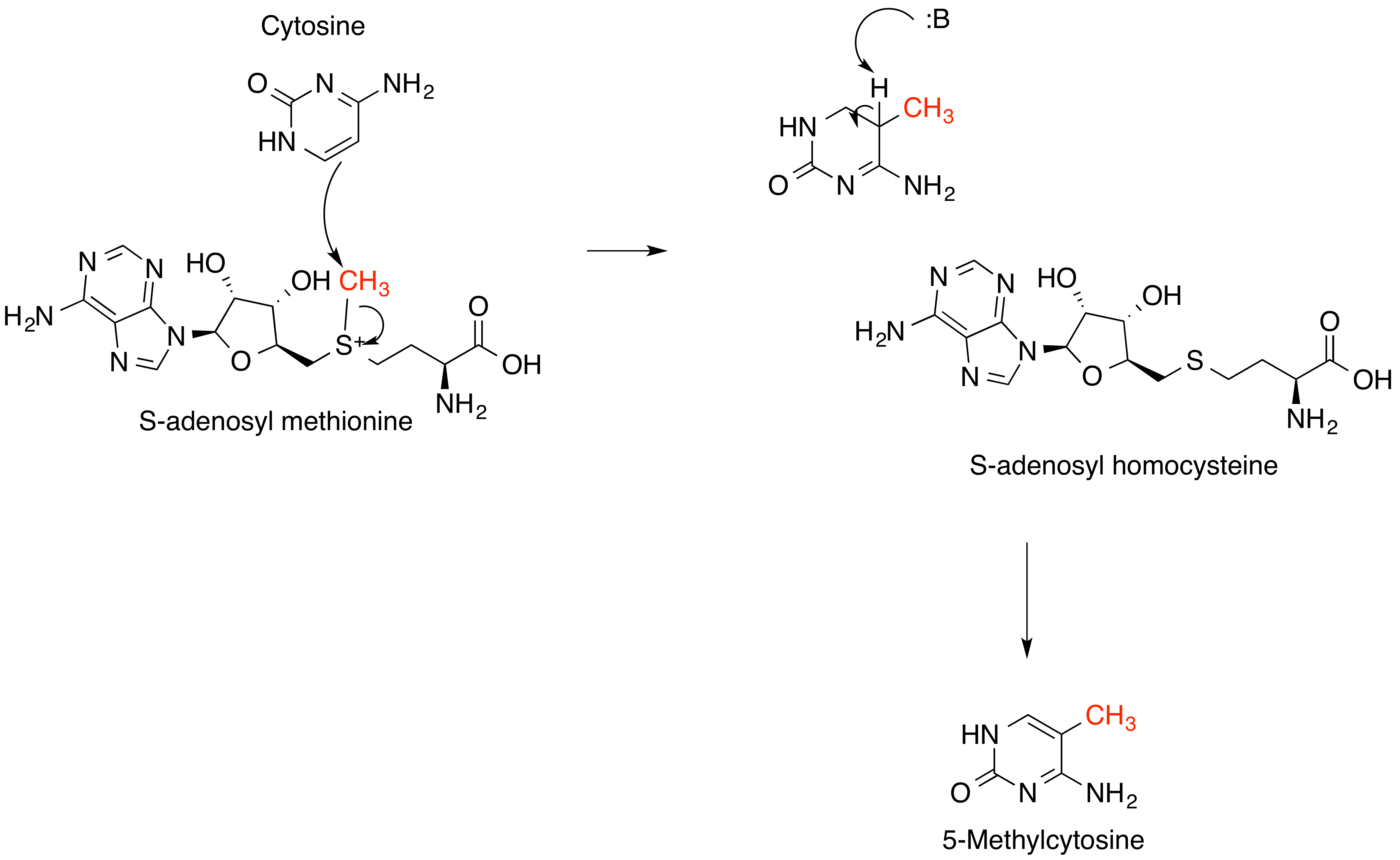
S-Adenosylmethionine
''S''-Adenosyl methionine (SAM), also known under the commercial names of SAMe, SAM-e, or AdoMet, is a common cosubstrate involved in methyl group transfers, transsulfuration, and aminopropylation. Although these anabolic reactions occur throughout the body, most SAM is produced and consumed in the liver. More than 40 methyl transfers from SAM are known, to various substrates such as nucleic acids, proteins, lipids and secondary metabolites. It is made from adenosine triphosphate (ATP) and methionine by methionine adenosyltransferase. SAM was first discovered by Giulio Cantoni in 1952. In bacteria, SAM is bound by the SAM riboswitch, which regulates genes involved in methionine or cysteine biosynthesis. In eukaryotic cells, SAM serves as a regulator of a variety of processes including DNA, tRNA, and rRNA methylation; immune response; amino acid metabolism; transsulfuration; and more. In plants, SAM is crucial to the biosynthesis of ethylene, an important plant hormone and si ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |