Johnson–Corey–Chaykovsky Reaction on:
[Wikipedia]
[Google]
[Amazon]
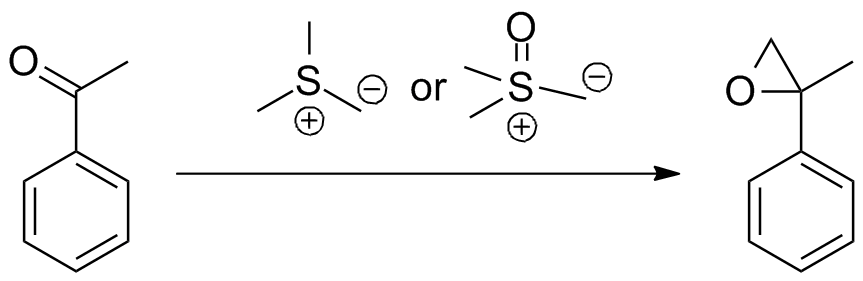
The Johnson–Corey–Chaykovsky reaction (sometimes referred to as the Corey–Chaykovsky reaction or CCR) is a chemical reaction used in organic chemistry for the synthesis of epoxides, aziridines, and  The reaction is most often employed for epoxidation via methylene transfer, and to this end has been used in several notable
The reaction is most often employed for epoxidation via methylene transfer, and to this end has been used in several notable
 The subsequent development of (dimethyloxosulfaniumyl)methanide, (CH3)2SOCH2 and (dimethylsulfaniumyl)methanide, (CH3)2SCH2 (known as Corey–Chaykovsky reagents) by Corey and Chaykovsky as efficient methylene-transfer reagents established the reaction as a part of the organic canon.
The subsequent development of (dimethyloxosulfaniumyl)methanide, (CH3)2SOCH2 and (dimethylsulfaniumyl)methanide, (CH3)2SCH2 (known as Corey–Chaykovsky reagents) by Corey and Chaykovsky as efficient methylene-transfer reagents established the reaction as a part of the organic canon.
 The ''trans'' diastereoselectivity observed results from the reversibility of the initial addition, allowing equilibration to the favored ''anti'' betaine over the ''syn'' betaine. Initial addition of the ylide results in a betaine with adjacent charges; density functional theory calculations have shown that the rate-limiting step is rotation of the central bond into the conformer necessary for backside attack on the sulfonium.
The ''trans'' diastereoselectivity observed results from the reversibility of the initial addition, allowing equilibration to the favored ''anti'' betaine over the ''syn'' betaine. Initial addition of the ylide results in a betaine with adjacent charges; density functional theory calculations have shown that the rate-limiting step is rotation of the central bond into the conformer necessary for backside attack on the sulfonium.
 The degree of reversibility in the initial step (and therefore the diastereoselectivity) depends on four factors, with greater reversibility corresponding to higher selectivity:
# ''Stability of the substrate'' with higher stability leading to greater reversibility by favoring the starting material over the betaine.
# ''Stability of the ylide'' with higher stability similarly leading to greater reversibility.
#'' Steric hindrance in the betaine'' with greater hindrance leading to greater reversibility by disfavoring formation of the intermediate and slowing the rate-limiting rotation of the central bond.
#''Solvation of charges in the betaine'' by
The degree of reversibility in the initial step (and therefore the diastereoselectivity) depends on four factors, with greater reversibility corresponding to higher selectivity:
# ''Stability of the substrate'' with higher stability leading to greater reversibility by favoring the starting material over the betaine.
# ''Stability of the ylide'' with higher stability similarly leading to greater reversibility.
#'' Steric hindrance in the betaine'' with greater hindrance leading to greater reversibility by disfavoring formation of the intermediate and slowing the rate-limiting rotation of the central bond.
#''Solvation of charges in the betaine'' by
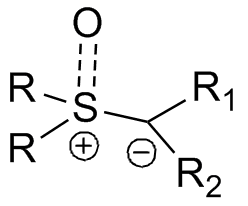 Many types of ylides can be prepared with various functional groups both on the anionic carbon center and on the sulfur. The substitution pattern can influence the ease of preparation for the reagents (typically from the sulfonium halide, e.g. trimethylsulfonium iodide) and overall reaction rate in various ways. The general format for the reagent is shown on the right.
Use of a sulfoxonium allows more facile preparation of the reagent using weaker bases as compared to sulfonium ylides. (The difference being that a sulfoxonium contains a doubly bonded oxygen whereas the sulfonium does not.) The former react slower due to their increased stability. In addition, the dialkylsulfoxide
Many types of ylides can be prepared with various functional groups both on the anionic carbon center and on the sulfur. The substitution pattern can influence the ease of preparation for the reagents (typically from the sulfonium halide, e.g. trimethylsulfonium iodide) and overall reaction rate in various ways. The general format for the reagent is shown on the right.
Use of a sulfoxonium allows more facile preparation of the reagent using weaker bases as compared to sulfonium ylides. (The difference being that a sulfoxonium contains a doubly bonded oxygen whereas the sulfonium does not.) The former react slower due to their increased stability. In addition, the dialkylsulfoxide

 The reaction has been used in a number of notable total syntheses including the
The reaction has been used in a number of notable total syntheses including the 



 *Several cycloadditions wherein the ylide serves as a " nucleophilic carbenoid equivalent" have been reported.
*Several cycloadditions wherein the ylide serves as a " nucleophilic carbenoid equivalent" have been reported.
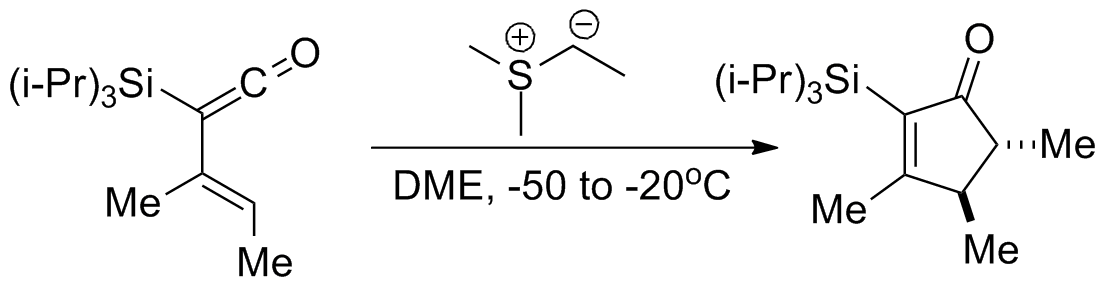 * Living polymerizations using trialkylboranes as the catalyst and (dimethyloxosulfaniumyl)methanide as the monomer have been reported for the synthesis of various complex polymers.
* Living polymerizations using trialkylboranes as the catalyst and (dimethyloxosulfaniumyl)methanide as the monomer have been reported for the synthesis of various complex polymers.

 The other major reagent is a
The other major reagent is a 
 Aggarwal has developed an alternative method employing the same sulfide as above and a novel alkylation involving a rhodium carbenoid formed '' in situ''. The method too has limited substrate scope, failing for any electrophiles possessing basic substituents due to
Aggarwal has developed an alternative method employing the same sulfide as above and a novel alkylation involving a rhodium carbenoid formed '' in situ''. The method too has limited substrate scope, failing for any electrophiles possessing basic substituents due to 
Animation of the mechanism
{{DEFAULTSORT:Johnson-Corey-Chaykovsky reaction Addition reactions Carbon-carbon bond forming reactions Epoxidation reactions Name reactions
cyclopropane
Cyclopropane is the cycloalkane with the molecular formula (CH2)3, consisting of three methylene groups (CH2) linked to each other to form a ring. The small size of the ring creates substantial ring strain in the structure. Cyclopropane itself ...
s. It was discovered in 1961 by A. William Johnson and developed significantly by E. J. Corey
Elias James Corey (born July 12, 1928) is an American organic chemist. In 1990, he won the Nobel Prize in Chemistry "for his development of the theory and methodology of organic synthesis", specifically retrosynthetic analysis. Regarded by many a ...
and Michael Chaykovsky. The reaction involves addition of a sulfur ylide to a ketone
In organic chemistry, a ketone is a functional group with the structure R–C(=O)–R', where R and R' can be a variety of carbon-containing substituents. Ketones contain a carbonyl group –C(=O)– (which contains a carbon-oxygen double bo ...
, aldehyde, imine, or enone to produce the corresponding 3-membered ring. The reaction is diastereoselective favoring ''trans'' substitution in the product regardless of the initial stereochemistry
Stereochemistry, a subdiscipline of chemistry, involves the study of the relative spatial arrangement of atoms that form the structure of molecules and their manipulation. The study of stereochemistry focuses on the relationships between stereois ...
. The synthesis of epoxides via this method serves as an important retrosynthetic alternative to the traditional epoxidation reactions of olefin
In organic chemistry, an alkene is a hydrocarbon containing a carbon–carbon double bond.
Alkene is often used as synonym of olefin, that is, any hydrocarbon containing one or more double bonds.H. Stephen Stoker (2015): General, Organic, an ...
s.
 The reaction is most often employed for epoxidation via methylene transfer, and to this end has been used in several notable
The reaction is most often employed for epoxidation via methylene transfer, and to this end has been used in several notable total syntheses
Total synthesis is the complete chemical synthesis of a complex molecule, often a natural product, from simple, commercially-available precursors. It usually refers to a process not involving the aid of biological processes, which distinguishes ...
(See Synthesis of epoxides below). Additionally detailed below are the history, mechanism, scope, and enantioselective variants of the reaction. Several reviews have been published.
History
The original publication by Johnson concerned the reaction of 9-dimethylsulfonium fluorenylide with substitutedbenzaldehyde
Benzaldehyde (C6H5CHO) is an organic compound consisting of a benzene ring with a formyl substituent. It is the simplest aromatic aldehyde and one of the most industrially useful.
It is a colorless liquid with a characteristic almond-like odor. ...
derivatives. The attempted Wittig-like reaction failed and a benzalfluorene oxide was obtained instead, noting that "Reaction between the sulfur ylid and benzaldehydes did not afford benzalfluorenes as had the phosphorus and arsenic ylids."
 The subsequent development of (dimethyloxosulfaniumyl)methanide, (CH3)2SOCH2 and (dimethylsulfaniumyl)methanide, (CH3)2SCH2 (known as Corey–Chaykovsky reagents) by Corey and Chaykovsky as efficient methylene-transfer reagents established the reaction as a part of the organic canon.
The subsequent development of (dimethyloxosulfaniumyl)methanide, (CH3)2SOCH2 and (dimethylsulfaniumyl)methanide, (CH3)2SCH2 (known as Corey–Chaykovsky reagents) by Corey and Chaykovsky as efficient methylene-transfer reagents established the reaction as a part of the organic canon.

Mechanism
Thereaction mechanism
In chemistry, a reaction mechanism is the step by step sequence of elementary reactions by which overall chemical change occurs.
A chemical mechanism is a theoretical conjecture that tries to describe in detail what takes place at each stage of ...
for the Johnson–Corey–Chaykovsky reaction consists of nucleophilic addition
In organic chemistry, a nucleophilic addition reaction is an addition reaction where a chemical compound with an electrophilic double or triple bond reacts with a nucleophile, such that the double or triple bond is broken. Nucleophilic additions di ...
of the ylide to the carbonyl or imine group. A negative charge is transferred to the heteroatom and because the sulfonium
In organic chemistry, a sulfonium ion, also known as sulphonium ion or sulfanium ion, is a positively-charged ion (a " cation") featuring three organic substituents attached to sulfur. These organosulfur compounds have the formula . Together wi ...
cation
An ion () is an atom or molecule with a net electrical charge.
The charge of an electron is considered to be negative by convention and this charge is equal and opposite to the charge of a proton, which is considered to be positive by convent ...
is a good leaving group it gets expelled forming the ring. In the related Wittig reaction, the formation of the much stronger phosphorus- oxygen double bond prevents oxirane formation and instead, olefination takes place through a 4-membered cyclic intermediate.
 The degree of reversibility in the initial step (and therefore the diastereoselectivity) depends on four factors, with greater reversibility corresponding to higher selectivity:
# ''Stability of the substrate'' with higher stability leading to greater reversibility by favoring the starting material over the betaine.
# ''Stability of the ylide'' with higher stability similarly leading to greater reversibility.
#'' Steric hindrance in the betaine'' with greater hindrance leading to greater reversibility by disfavoring formation of the intermediate and slowing the rate-limiting rotation of the central bond.
#''Solvation of charges in the betaine'' by
The degree of reversibility in the initial step (and therefore the diastereoselectivity) depends on four factors, with greater reversibility corresponding to higher selectivity:
# ''Stability of the substrate'' with higher stability leading to greater reversibility by favoring the starting material over the betaine.
# ''Stability of the ylide'' with higher stability similarly leading to greater reversibility.
#'' Steric hindrance in the betaine'' with greater hindrance leading to greater reversibility by disfavoring formation of the intermediate and slowing the rate-limiting rotation of the central bond.
#''Solvation of charges in the betaine'' by counterion
160px, Polystyrene sulfonate, a cation-exchange resin, is typically supplied with as the counterion.">cation-exchange_resin.html" ;"title="Polystyrene sulfonate, a cation-exchange resin">Polystyrene sulfonate, a cation-exchange resin, is typical ...
s such as lithium with greater solvation allowing more facile rotation in the betaine intermediate, lowering the amount of reversibility.
Scope
The application of the Johnson–Corey–Chaykovsky reaction in organic synthesis is diverse. The reaction has come to encompass reactions of many types of sulfur ylides with electrophiles well beyond the original publications. It has seen use in a number of high-profile total syntheses, as detailed below, and is generally recognized as a powerful transformative tool in the organic repertoire.Types of ylides
 Many types of ylides can be prepared with various functional groups both on the anionic carbon center and on the sulfur. The substitution pattern can influence the ease of preparation for the reagents (typically from the sulfonium halide, e.g. trimethylsulfonium iodide) and overall reaction rate in various ways. The general format for the reagent is shown on the right.
Use of a sulfoxonium allows more facile preparation of the reagent using weaker bases as compared to sulfonium ylides. (The difference being that a sulfoxonium contains a doubly bonded oxygen whereas the sulfonium does not.) The former react slower due to their increased stability. In addition, the dialkylsulfoxide
Many types of ylides can be prepared with various functional groups both on the anionic carbon center and on the sulfur. The substitution pattern can influence the ease of preparation for the reagents (typically from the sulfonium halide, e.g. trimethylsulfonium iodide) and overall reaction rate in various ways. The general format for the reagent is shown on the right.
Use of a sulfoxonium allows more facile preparation of the reagent using weaker bases as compared to sulfonium ylides. (The difference being that a sulfoxonium contains a doubly bonded oxygen whereas the sulfonium does not.) The former react slower due to their increased stability. In addition, the dialkylsulfoxide by-product
A by-product or byproduct is a secondary product derived from a production process, manufacturing process or chemical reaction; it is not the primary product or service being produced.
A by-product can be useful and marketable or it can be consid ...
s of sulfoxonium reagents are greatly preferred to the significantly more toxic, volatile, and odorous dialkylsulfide by-products from sulfonium reagents.
The vast majority of reagents are monosubstituted at the ylide carbon (either R1 or R2 as hydrogen). Disubstituted reagents are much rarer but have been described:
#If the ylide carbon is substituted with an electron-withdrawing group
In chemistry, an electron-withdrawing group (EWG) is a substituent that has some of the following kinetic and thermodynamic implications:
*with regards to electron transfer, electron-withdrawing groups enhance the oxidizing power tendency of the ...
(EWG), the reagent is referred to as a ''stabilized ylide''. These, similarly to sulfoxonium reagents, react much slower and are typically easier to prepare. These are limited in their usefulness as the reaction can become prohibitively sluggish: examples involving amides are widespread, with many fewer involving esters and virtually no examples involving other EWG's. For these, the related Darzens reaction is typically more appropriate.
#If the ylide carbon is substituted with an aryl or allyl
In organic chemistry, an allyl group is a substituent with the structural formula , where R is the rest of the molecule. It consists of a methylene bridge () attached to a vinyl group (). The name is derived from the scientific name for garlic, ...
group, the reagent is referred to as a ''semi-stabilized ylide''. These have been developed extensively, second only to the classical methylene reagents (R1=R2=H). The substitution pattern on aryl reagents can heavily influence the selectivity of the reaction as per the criteria above.
#If the ylide carbon is substituted with an alkyl group the reagent is referred to as an ''unstabilized ylide''. The size of the alkyl groups are the major factors in selectivity with these reagents.
The R-groups on the sulfur, though typically methyl
In organic chemistry, a methyl group is an alkyl derived from methane, containing one carbon atom bonded to three hydrogen atoms, having chemical formula . In formulas, the group is often abbreviated as Me. This hydrocarbon group occurs in many ...
s, have been used to synthesize reagents that can perform enantioselective variants of the reaction (See Variations below). The size of the groups can also influence diastereoselectivity in alicyclic
In organic chemistry, an alicyclic compound contains one or more all-carbon rings which may be either saturated or unsaturated, but do not have aromatic character. Alicyclic compounds may have one or more aliphatic side chains attached.
The ...
substrates.
Synthesis of epoxides
Reactions of sulfur ylides withketone
In organic chemistry, a ketone is a functional group with the structure R–C(=O)–R', where R and R' can be a variety of carbon-containing substituents. Ketones contain a carbonyl group –C(=O)– (which contains a carbon-oxygen double bo ...
s and aldehydes to form epoxides are by far the most common application of the Johnson–Corey–Chaykovsky reaction. Examples involving complex substrates and 'exotic' ylides have been reported, as shown below.

 The reaction has been used in a number of notable total syntheses including the
The reaction has been used in a number of notable total syntheses including the Danishefsky Taxol total synthesis
The Danishefsky Taxol total synthesis in organic chemistry is an important third Taxol synthesis published by the group of Samuel Danishefsky in 1996 two years after the first two efforts described in the Holton Taxol total synthesis and the Nic ...
, which produces the chemotherapeutic drug taxol, and the Kuehne Strychnine total synthesis which produces the pesticide strychnine
Strychnine (, , US chiefly ) is a highly toxic, colorless, bitter, crystalline alkaloid used as a pesticide, particularly for killing small vertebrates such as birds and rodents. Strychnine, when inhaled, swallowed, or absorbed through the eye ...
.


Synthesis of aziridines
The synthesis of aziridines from imines is another important application of the Johnson–Corey–Chaykovsky reaction and provides an alternative to amine transfer from oxaziridines. Though less widely applied, the reaction has a similar substrate scope and functional group tolerance to the carbonyl equivalent. The examples shown below are representative; in the latter, an aziridine forms ''in situ'' and is opened via nucleophilic attack to form the corresponding amine.
Synthesis of cyclopropanes
For addition of sulfur ylides to enones, higher 1,4-selectivity is typically obtained with sulfoxonium reagents than with sulfonium reagents. Many electron-withdrawing groups have been shown compatible with the reaction includingketone
In organic chemistry, a ketone is a functional group with the structure R–C(=O)–R', where R and R' can be a variety of carbon-containing substituents. Ketones contain a carbonyl group –C(=O)– (which contains a carbon-oxygen double bo ...
s, esters, and amides (the example below involves a Weinreb amide). With further conjugated systems 1,6-addition tends to predominate over 1,4-addition.

Other reactions
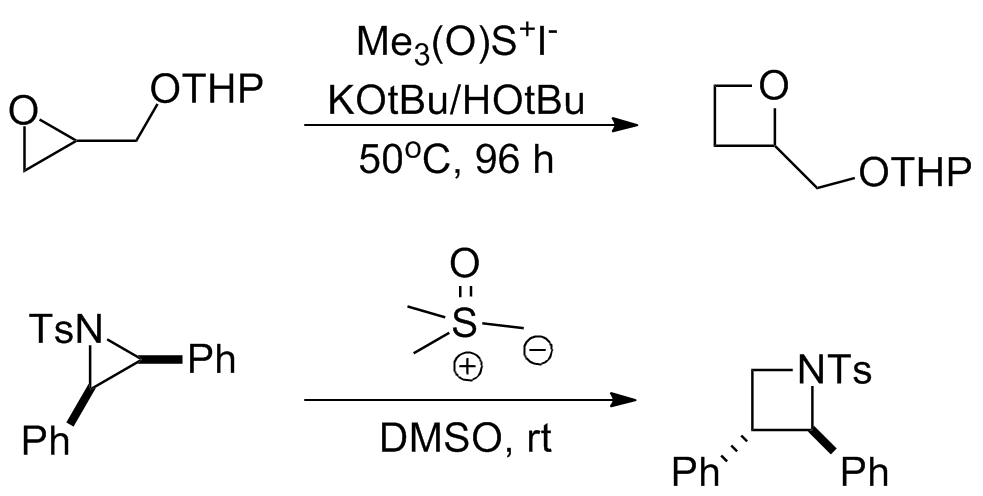
In addition to the reactions originally reported by Johnson, Corey, and Chaykovsky, sulfur ylides have been used for a number of related homologation reactions that tend to be grouped under the same name. *With epoxides and aziridines the reaction serves as a ring-expansion to produce the corresponding oxetane or azetidine. The long reaction times required for these reactions prevent them from occurring as significant side reactions when synthesizing epoxides and aziridines. *Several cycloadditions wherein the ylide serves as a " nucleophilic carbenoid equivalent" have been reported.
*Several cycloadditions wherein the ylide serves as a " nucleophilic carbenoid equivalent" have been reported.
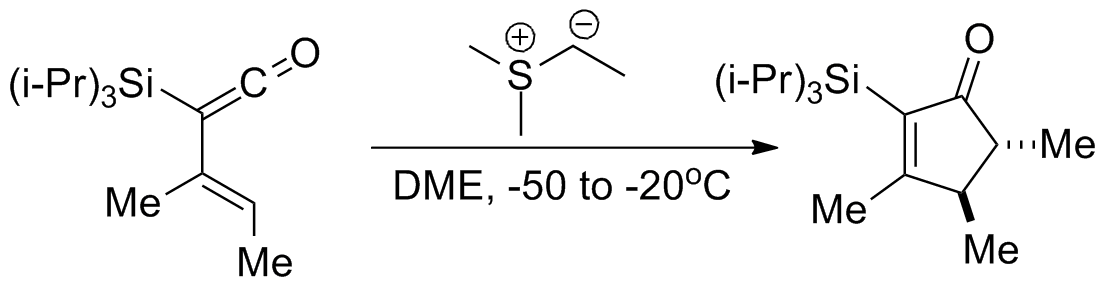 * Living polymerizations using trialkylboranes as the catalyst and (dimethyloxosulfaniumyl)methanide as the monomer have been reported for the synthesis of various complex polymers.
* Living polymerizations using trialkylboranes as the catalyst and (dimethyloxosulfaniumyl)methanide as the monomer have been reported for the synthesis of various complex polymers.

Enantioselective variations
The development of an enantioselective (i.e. yielding an enantiomeric excess, which is labelled as "ee") variant of the Johnson–Corey–Chaykovsky reaction remains an active area of academic research. The use of chiral sulfides in a stoichiometric fashion has proved more successful than the corresponding catalytic variants, but the substrate scope is still limited in all cases. The catalytic variants have been developed almost exclusively for enantioselective purposes; typical organosulfide reagents are not prohibitively expensive and the racemic reactions can be carried out with equimolar amounts of ylide without raising costs significantly. Chiral sulfides, on the other hand, are more costly to prepare, spurring the advancement of catalytic enantioselective methods.Stoichiometric reagents
The most successful reagents employed in a stoichiometric fashion are shown below. The first is a bicyclic oxathiane that has been employed in the synthesis of the β-adrenergic compound ''dichloroisoproterenol'' (DCI) but is limited by the availability of only one enantiomer of the reagent. The synthesis of theaxial
Axial may refer to:
* one of the anatomical directions describing relationships in an animal body
* In geometry:
:* a geometric term of location
:* an axis of rotation
* In chemistry, referring to an axial bond
* a type of modal frame, in music
* ...
diastereomer is rationalized via the 1,3- anomeric effect which reduces the nucleophilicity of the equatorial lone pair
In chemistry, a lone pair refers to a pair of valence electrons that are not shared with another atom in a covalent bondIUPAC ''Gold Book'' definition''lone (electron) pair''/ref> and is sometimes called an unshared pair or non-bonding pair. Lone ...
. The conformation of the ylide is limited by transannular strain and approach of the aldehyde is limited to one face of the ylide by steric interactions with the methyl substituents.
 The other major reagent is a
The other major reagent is a camphor
Camphor () is a waxy, colorless solid with a strong aroma. It is classified as a terpenoid and a cyclic ketone. It is found in the wood of the camphor laurel ('' Cinnamomum camphora''), a large evergreen tree found in East Asia; and in the k ...
-derived reagent developed by Varinder Aggarwal of the University of Bristol. Both enantiomer
In chemistry, an enantiomer ( /ɪˈnænti.əmər, ɛ-, -oʊ-/ ''ih-NAN-tee-ə-mər''; from Ancient Greek ἐνάντιος ''(enántios)'' 'opposite', and μέρος ''(méros)'' 'part') – also called optical isomer, antipode, or optical ant ...
s are easily synthesized, although the yields are lower than for the oxathiane reagent. The ylide conformation is determined by interaction with the bridgehead hydrogens and approach of the aldehyde is blocked by the camphor moiety
Moiety may refer to:
Chemistry
* Moiety (chemistry), a part or functional group of a molecule
** Moiety conservation, conservation of a subgroup in a chemical species
Anthropology
* Moiety (kinship), either of two groups into which a society is ...
. The reaction employs a phosphazene base to promote formation of the ylide.

Catalytic reagents
Catalytic reagents have been less successful, with most variations suffering from poor yield, poor enantioselectivity, or both. There are also issues with substrate scope, most having limitations with methylene transfer and aliphatic aldehydes. The trouble stems from the need for a nucleophilic sulfide that efficiently generates the ylide which can also act as a good leaving group to form the epoxide. Since the factors underlying these desiderata are at odds, tuning of the catalyst properties has proven difficult. Shown below are several of the most successful catalysts along with the yields and enantiomeric excess for their use in synthesis of(E)-stilbene
(''E'')-Stilbene, commonly known as ''trans''-stilbene, is an organic compound represented by the condensed structural formula CHCH=CHCH. Classified as a diarylethene, it features a central ethylene moiety with one phenyl group substituent on ...
oxide.
 Aggarwal has developed an alternative method employing the same sulfide as above and a novel alkylation involving a rhodium carbenoid formed '' in situ''. The method too has limited substrate scope, failing for any electrophiles possessing basic substituents due to
Aggarwal has developed an alternative method employing the same sulfide as above and a novel alkylation involving a rhodium carbenoid formed '' in situ''. The method too has limited substrate scope, failing for any electrophiles possessing basic substituents due to competitive consumption
In sociology and in economics, the term conspicuous consumption describes and explains the consumer practice of buying and using goods of a higher quality, price, or in greater quantity than practical. In 1899, the sociologist Thorstein Veblen co ...
of the carbenoid.

See also
* Darzens reaction * Wittig reaction * Epoxidation * Ylide *E.J. Corey
Elias James Corey (born July 12, 1928) is an American organic chemist. In 1990, he won the Nobel Prize in Chemistry "for his development of the theory and methodology of organic synthesis", specifically retrosynthetic analysis. Regarded by many ...
* Taxol total synthesis
* Strychnine total synthesis
References
External links
Animation of the mechanism
{{DEFAULTSORT:Johnson-Corey-Chaykovsky reaction Addition reactions Carbon-carbon bond forming reactions Epoxidation reactions Name reactions