|
Phosphazene
Phosphazenes refer to various classes of organophosphorus compounds featuring phosphorus(V) with a double bond between P and N. One class of phosphazenes have the formula . These phosphazenes are also known as iminophosphoranes and phosphine imides. They are superbases. BEMP and ''t''-Bu-P4 Well known phosphazene bases are BEMP (2-tert-Butylimino-2-diEthylamino-1,3-diMethylperhydro-1,3,2-diazaPhosphorine) with an acetonitrile pKa, p''K''a of the conjugate acid of 27.6 and the phosphorimidic triamide P4-t-Bu, ''t''-Bu-P4 (p''K''BH+ = 42.7) also known as P4-t-Bu, Schwesinger base. BEMP and P4-t-Bu, ''t''-Bu-P4 have attracted attention because they are nucleophilicity, low-nucleophilic, which precludes their participating in competing reactions. Being non-ionic ("charge-neutral"), they are soluble in nonpolar solvents. Protonation takes place at a doubly bonded nitrogen atom. The p''K''a's of , where R = Methyl group, Me and Pyrrolidine, pyrrolidinyl, are 42.7 and 44, respectively. ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Polyphosphazene
Polyphosphazenes include a wide range of hybrid inorganic chemistry, inorganic-organic chemistry, organic polymers with a number of different polymer architecture, skeletal architectures with the backbone phosphorus, P-nitrogen, N-P-N-P-N-. In nearly all of these materials two organic side groups are attached to each phosphorus center. Linear polymers have the formula (N=PR1R2)n, where R1 and R2 are organic (see graphic). Other architectures are cyclolinear and cyclomatrix polymers in which small phosphazene, phosphazene rings are connected together by organic chain units. Other architectures are available, such as block copolymer, polymer architecture, star, dendritic polymer, dendritic, or polymer architecture, comb-type structures. More than 700 different polyphosphazenes are known, with different side groups (R) and different molecular architectures. Many of these polymers were first synthesized and studied in the research group of Harry R. Allcock. __TOC__ Synthesis The method ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
P4-t-Bu
P4-''t''-Bu is a readily accessible chemical from the group of neutral, peralkylated Steric effects#Steric hindrance, sterically hindered polyaminophosphazenes, which are extremely strong Base (chemistry), bases but very weak nucleophiles, with the formula . "''t''-Bu" stands for Butyl group, ''tert''-butyl –. "P4" stands for the fact that this molecule has 4 phosphorus atoms. P4-''t''-Bu can also be regarded as tetrameric triaminoiminophosphorane of the basic structure . The homologous series of P1 to P7 polyaminophosphazenes of the general formula [(R^1_2N)_3P=N-]_\mathit-(R^1_2N)_P=NR2 with preferably methyl groups as R1, a methyl group or tert-butyl group, ''tert''-butyl group as and even-numbered ''x'' between 0 and 6 (P4-''t''-Bu: R1 = Me, R2 = ''t''-Bu and ''x'' = 3) has been developed by Reinhard Schwesinger; the resulting phosphazene bases are therefore also referred to as Schwesinger superbases. Preparation The convergent synthesis of P4-''t''-Bu is derived from phosph ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Superbases
A superbase is a Chemical compound, compound that has a particularly high affinity for Hydron (chemistry), protons. Superbases are of theoretical interest and potentially valuable in organic synthesis. Superbases have been described and used since the 1850s.''Superbases for Organic Synthesis'' Ed. Ishikawa, T., John Wiley and Sons, Ltd.: West Sussex, UK. 2009. Definitions Generically International Union of Pure and Applied Chemistry, IUPAC defines a superbase as a "compound having a very high Base (chemistry), basicity, such as lithium diisopropylamide." Superbases are often defined in two broad categories, Organic chemistry, organic and Organometallic chemistry, organometallic. Organic superbases are charge-neutral compounds with basicities greater than that of proton sponge (1,8-bis(dimethylamino)naphthalene, pKBH+ = 18.6 in acetonitrile). In a related definition: any species with a higher absolute proton affinity (APA = 245.3 kcal/mol) and intrinsic gas phase basicity (GB = 23 ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Superbase
A superbase is a compound that has a particularly high affinity for protons. Superbases are of theoretical interest and potentially valuable in organic synthesis. Superbases have been described and used since the 1850s.''Superbases for Organic Synthesis'' Ed. Ishikawa, T., John Wiley and Sons, Ltd.: West Sussex, UK. 2009. Definitions Generically IUPAC defines a superbase as a "compound having a very high basicity, such as lithium diisopropylamide." Superbases are often defined in two broad categories, organic and organometallic. Organic superbases are charge-neutral compounds with basicities greater than that of proton sponge (1,8-bis(dimethylamino)naphthalene, pKBH+ = 18.6 in acetonitrile). In a related definition: any species with a higher absolute proton affinity (APA = 245.3 kcal/mol) and intrinsic gas phase basicity (GB = 239 kcal/mol) than proton sponge. Common superbases of this variety feature amidine, guanidine, and phosphazene functional groups. Strong superbases c ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
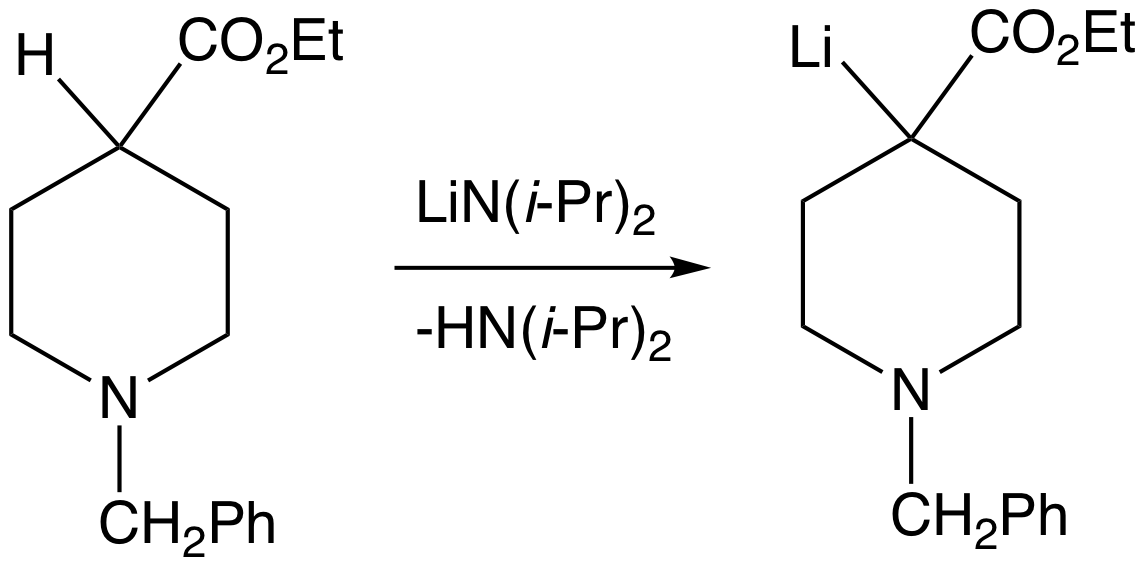
Non-nucleophilic Bases
As the name suggests, a non-nucleophilic base is a sterically hindered organic base that is a poor nucleophile. Normal bases are also nucleophiles, but often chemists seek the proton-removing ability of a base without any other functions. Typical non-nucleophilic bases are bulky, such that protons can attach to the basic center but alkylation and complexation is inhibited. Non-nucleophilic bases A variety of amines and nitrogen heterocycles are useful bases of moderate strength (pKa of conjugate acid around 10-13) * ''N'',''N''-Diisopropylethylamine (DIPEA, also called Hünig's Base), pKa = 10.75 *1,8-Diazabicycloundec-7-ene (DBU) - useful for E2 elimination reactions, pKa = 13.5 * 1,5-Diazabicyclo(4.3.0)non-5-ene (DBN) - comparable to DBU * 2,6-Di-tert-butylpyridine, a weak non-nucleophilic base pKa = 3.58 * Phosphazene bases, such as ''t''-Bu-P4''Activation in anionic polymerization: Why phosphazene bases are very exciting promoters'' S. Boileau, N. Illy Prog. Polym. Sci., 20 ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Hexachlorophosphazene
Hexachlorophosphazene is an inorganic compound with the chemical formula . The molecule has a cyclic, unsaturated backbone consisting of alternating phosphorus and nitrogen atoms, and can be viewed as a trimer of the hypothetical compound (phosphazyl dichloride). Its classification as a phosphazene highlights its relationship to benzene. There is large academic interest in the compound relating to the phosphorus-nitrogen bonding and phosphorus reactivity. Occasionally, commercial or suggested practical applications have been reported, too, utilising hexachlorophosphazene as a precursor chemical.Mark, J. E.; Allcock, H. R.; West, R. “Inorganic Polymers” Prentice Hall, Englewood, NJ: 1992. . Derivatives of noted interest include the hexalkoxyphosphazene lubricants obtained from nucleophilic substitution of hexachlorophosphazene with alkoxides, or chemically resistant inorganic polymers with desirable thermal and mechanical properties known as polyphosphazenes produced from ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Cyclodiphosphazane
Cyclodiphosphazanes are saturated four membered P2N2 ring systems and one of the major classes of cyclic phosphazene compounds. Bis(chloro)cyclodiphosphazanes, (cis- lP(μ-NR)sub>2) are important starting compounds for synthesizing a variety of cyclodiphosphazane derivatives by nucleophilic substitution reactions; are prepared by reaction of phosphorus trichloride (PCl3) with a primary amine (RNH2) or amine hydrochlorides (RNH3Cl). Organic substituents on nitrogen play an important role in formation of cyclic phosphazane compounds. The cyclic tetramers and trimer are formed with methyl and ethyl substituents on nitrogen, whereas formation of cyclic dimers (cis- lP(μ-NR)sub>2) have been observed exclusively with more sterically demanding primary amines such as ''tert''-butylamine and aniline. Coordination Chemistry Cyclodiphosphazanes are excellent ligand systems for metallosupramolecular chemistry. The cis-oriented lone pair on phosphorus in cyclodiphosphazane are projected away ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Verkade Base
In chemistry, the Verkade base (or Verkade superbase) is a powerful superbase with the formula P(MeNCH2CH2)3N. A colorless oil, it is an aminophosphine although its inventor John Verkade called it proazaphosphatrane. The trimethyl derivative or 2,5,8,9-tetraaza-1-phosphabicyclo .3.3ndecane is the simplest. Diverse analogues of the Verkade base are known, e.g. with isopropyl groups in place of methyl. Synthesis and reactions The Verkade base is generated by the reaction of N,N,N-trimethyl tren with tris(dimethylamino)phosphine: :P(NMe2)3 + (MeNHCH2CH2)3N → P(MeNCH2CH2)3N + 3 Me2NH The principal reaction of the Verkade base is protonation. The proton is attacked by the Verkade base at the phosphorus atom within, which induces the formation of a transannular P-N bond. The product exemplifies the structure of an atrane. 290 px, left, Protonation of Verkade base. The conjugate acid P(MeNCH2CH2)3Nsup>+ of the base has a pKa of 32.9 in acetonitrile. For comparison, th ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Bis(triphenylphosphine)iminium Chloride
Bis(triphenylphosphine)iminium chloride is the chemical compound with the formula , often abbreviated , where Ph is phenyl , or even abbreviated [PPN]Cl or [PNP]Cl or PPNCl or PNPCl, where PPN or PNP stands for . This colorless salt is a source of the cation (abbreviated or ), which is used as an unreactive and weakly coordinating cation to isolate reactive anions. is a phosphazene. Synthesis and structure is prepared in two steps from triphenylphosphine : : This triphenylphosphine dichloride is related to phosphorus pentachloride . Treatment of this species with hydroxylamine in the presence of results in replacement of the two single P–Cl bonds in by one double P=N bond: : Triphenylphosphine oxide is a by-product. Bis(triphenylphosphine)iminium chloride is described as . The structure of the bis(triphenylphosphine)iminium chloride is . The P=N=P angle in the cation is flexible, ranging from ~130 to 180° depending on the salt. Bent and linear forms of the P=N=P conn ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Phosphine Imide
In chemistry a phosphine imide (sometimes abbreviated to phosphinimide) also known as a iminophosphorane is a functional group with the formula R3P=NR. While structurally related to phosphine oxide its chemistry has more in common with phosphonium ylides. Anions of this group, with the structure R3P=N−, are called phosphinoimidates and are used as ligands to form phosphinimide complexes which are highly active catalysts in some olefin polymerization reactions. Synthesis Phosphine imides can be isolated as intermediates in the Staudinger reaction and have also been prepared by the action of hydroxylamine-O-sulfonic acid on phosphines, proceeding via a p-aminophosphonium salt. Reactions and applications The functional group will readily hydrolyse to give a phosphine oxide and an amine :R3P=NR' + H2O → R3P=O + R'NH2 Phosphinimide ligands of the general formula NPR3− form transition metal phosphinimide complexeses. Some of these complexes are potential cataly ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Amide
In organic chemistry, an amide, also known as an organic amide or a carboxamide, is a chemical compound, compound with the general formula , where R, R', and R″ represent any group, typically organyl functional group, groups or hydrogen atoms. The amide group is called a peptide bond when it is part of the Polymer backbone, main chain of a protein, and an isopeptide bond when it occurs in a side chain, as in asparagine and glutamine. It can be viewed as a Derivative (chemistry), derivative of a carboxylic acid () with the hydroxyl group () replaced by an amino group (); or, equivalently, an acyl group, acyl (alkanoyl) group () joined to an amino group. Common amides are formamide (), acetamide (), benzamide (), and dimethylformamide (). Some uncommon examples of amides are ''N''-chloroacetamide () and chloroformamide (). Amides are qualified as primary (chemistry), primary, secondary (chemistry), secondary, and tertiary (chemistry), tertiary according to the number of acyl ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |