|
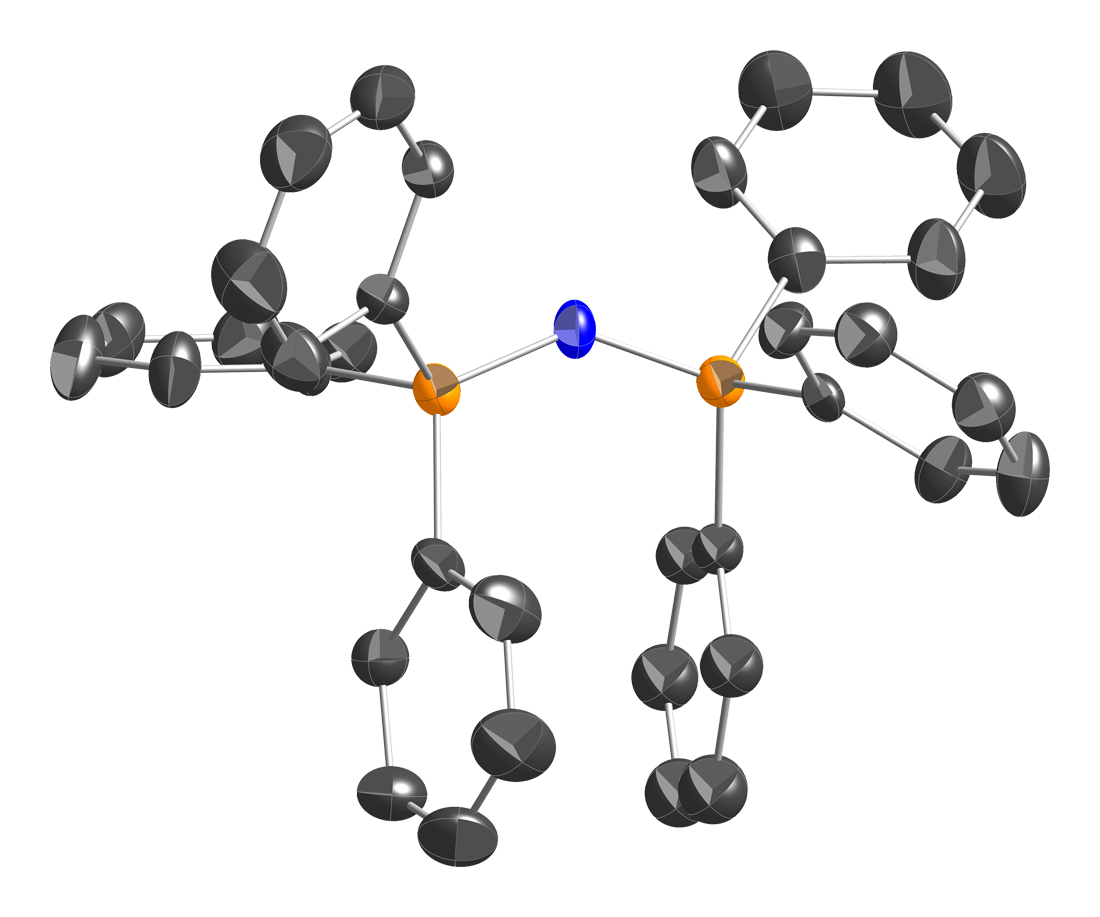
Bis(triphenylphosphine)iminium Chloride
Bis(triphenylphosphine)iminium chloride is the chemical compound with the formula , often abbreviated , where Ph is phenyl , or even abbreviated PNl or NPl or PPNCl or PNPCl, where PPN or PNP stands for . This colorless salt is a source of the cation (abbreviated or ), which is used as an unreactive and weakly coordinating cation to isolate reactive anions. is a phosphazene. Synthesis and structure is prepared in two steps from triphenylphosphine : : This triphenylphosphine dichloride is related to phosphorus pentachloride . Treatment of this species with hydroxylamine in the presence of results in replacement of the two single P–Cl bonds in by one double P=N bond: : Triphenylphosphine oxide is a by-product. Bis(triphenylphosphine)iminium chloride is described as . The structure of the bis(triphenylphosphine)iminium cation is . The P=N=P angle in the cation is flexible, ranging from ~130 to 180° depending on the salt. Bent and linear forms of the P=N=P connections ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Tetraphenylarsonium Chloride
Tetraphenylarsonium chloride is the organoarsenic compound with the formula (C6H5)4AsCl. This white solid is the chloride salt of the tetraphenylarsonium cation, which is tetrahedral. Typical of related quat salts, it is soluble in polar organic solvents. It often is used as a hydrate. Synthesis and reactions It is prepared by neutralization of tetraphenylarsonium chloride hydrochloride, which is produced from triphenylarsine: :(C6H5)3As + Br2 → (C6H5)3AsBr2 :(C6H5)3AsBr2 + H2O → (C6H5)3AsO + 2 HBr : (C6H5)3AsO + C6H5MgBr → (C6H5)4AsOMgBr :(C6H5)4AsOMgBr + 3 HCl → (C6H5)4AsCl.HCl + MgBrCl :(C6H5)4AsCl.HCl + NaOH → (C6H5)4AsCl + NaCl + H2O Like other quat salts, it is used to solubilize polyatomic anions in organic media.{{cite book , doi=10.1002/9780470132470.ch36, chapter=Tetraethylammonium, Tetraphenylarsonium, and Ammonium Cyanates and Cyanides, year=1976, volume=16, last1=Dieck, first1=R. L., last2=Peterson, first2=E. J., last3=Gallia ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Hexaphenylcarbodiphosphorane
Hexaphenylcarbodiphosphorane is the organophosphorus compound with the formula C(PPh3)2 (where Ph = C6H5). It is a yellow, moisture-sensitive solid. The compound is classified as an ylide and as such carries significant negative charge on carbon. It is isoelectronic with bis(triphenylphosphine)iminium. The P-C-P angle is 131°. The compound has attracted attention as an unusual ligand in organometallic chemistry. The pure compound has two crystalline phases: a metastable monoclinic C2 phase that is triboluminescent, and an orthorhombic P222 form that is not. Both polymorphs are photoluminescent, with respective peak wavelengths at 540 and 575 nm. Preparation The compound was originally prepared by deprotonation of the phosphonium salt C(PPh3)2r using potassium. An improved procedure entails production of the same double phosphonium salt from methylene bromide. The double deprotonation is effected with potassium amide.{{cite journal , doi=10.1016/j.ica.2017.04.018, ti ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Chlorides
The chloride ion is the anion (negatively charged ion) Cl−. It is formed when the element chlorine (a halogen) gains an electron or when a compound such as hydrogen chloride is dissolved in water or other polar solvents. Chloride salts such as sodium chloride are often very soluble in water.Green, John, and Sadru Damji. "Chapter 3." ''Chemistry''. Camberwell, Vic.: IBID, 2001. Print. It is an essential electrolyte located in all body fluids responsible for maintaining acid/base balance, transmitting nerve impulses and regulating liquid flow in and out of cells. Less frequently, the word ''chloride'' may also form part of the "common" name of chemical compounds in which one or more chlorine atoms are covalently bonded. For example, methyl chloride, with the standard name chloromethane (see IUPAC books) is an organic compound with a covalent C−Cl bond in which the chlorine is not an anion. Electronic properties A chloride ion (diameter 167 pm) is much larger than ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Organophosphorus Compounds
Organophosphorus compounds are organic compounds containing phosphorus. They are used primarily in pest control as an alternative to chlorinated hydrocarbons that persist in the environment. Some organophosphorus compounds are highly effective insecticides, although some are extremely toxic to humans, including sarin and VX (nerve agent), VX nerve agents. Organophosphorus chemistry is the corresponding science of the properties and reactivity of organophosphorus compounds. Phosphorus, like nitrogen, is in pnictogen, group 15 of the periodic table, and thus phosphorus compounds and nitrogen compounds have many similar properties. The definition of organophosphorus compounds is variable, which can lead to confusion. In industrial and environmental chemistry, an organophosphorus compound need contain only an organic substituent, but need not have a direct phosphorus-carbon (P-C) bond. Thus a large proportion of pesticides (e.g., malathion), are often included in this class of compound ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Ion Pair
In chemistry, ion association is a chemical reaction whereby ions of opposite electric charge come together in solution to form a distinct chemical entity. Ion associates are classified, according to the number of ions that associate with each other, as ion pairs, ion triplets, etc. Ion pairs are also classified according to the nature of the interaction as contact, solvent-shared or solvent-separated. The most important factor to determine the extent of ion association is the dielectric constant of the solvent. Ion associates have been characterized by means of vibrational spectroscopy, as introduced by Niels Bjerrum, and dielectric-loss spectroscopy. Classification of ion pairs ''Ion pairs'' are formed when a cation and anion, which are present in a solution of an ionizable substance, come together to form a discrete chemical species. There are three distinct types of ''ion pairs'', depending on the extent of solvation of the two ions. For example, magnesium sulphate exist ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Alkali Metal
The alkali metals consist of the chemical elements lithium (Li), sodium (Na), potassium (K),The symbols Na and K for sodium and potassium are derived from their Latin names, ''natrium'' and ''kalium''; these are still the origins of the names for the elements in some languages, such as German and Russian. rubidium (Rb), caesium (Cs), and francium (Fr). Together with hydrogen they constitute Group (periodic table)#Group names, group 1, which lies in the s-block of the periodic table. All alkali metals have their outermost electron in an atomic orbital, s-orbital: this shared electron configuration results in their having very similar characteristic properties. Indeed, the alkali metals provide the best example of periodic trends, group trends in properties in the periodic table, with elements exhibiting well-characterised homology (chemistry), homologous behaviour. This family of elements is also known as the lithium family after its leading element. The alkali metals are all sh ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Quaternary Ammonium
In chemistry, quaternary ammonium cations, also known as quats, are positively charged polyatomic ions of the structure , R being an alkyl group or an aryl group. Unlike the ammonium ion () and the primary, secondary, or tertiary ammonium cations, the quaternary ammonium cations are permanently charged, independent of the pH of their solution. Quaternary ammonium salts or quaternary ammonium compounds (called quaternary amines in oilfield parlance) are salts of quaternary ammonium cations. Polyquats are a variety of engineered polymer forms which provide multiple quat molecules within a larger molecule. Quats are used in consumer applications including as antimicrobials (such as detergents and disinfectants), fabric softeners, and hair conditioners. As an antimicrobial, they are able to inactivate enveloped viruses (such as SARS-CoV-2). Quats tend to be gentler on surfaces than bleach-based disinfectants, and are generally fabric-safe. Synthesis Quaternary ammonium comp ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Azide
In chemistry, azide is a linear, polyatomic anion with the formula and structure . It is the conjugate base of hydrazoic acid . Organic azides are organic compounds with the formula , containing the azide functional group. The dominant application of azides is as a propellant in air bags. Preparation Sodium azide is made industrially by the reaction of nitrous oxide, with sodium amide in liquid ammonia as solvent: : Many inorganic azides can be prepared directly or indirectly from sodium azide. For example, lead azide, used in detonators, may be prepared from the metathesis reaction between lead nitrate and sodium azide. An alternative route is direct reaction of the metal with silver azide dissolved in liquid ammonia. Some azides are produced by treating the carbonate salts with hydrazoic acid. Bonding Azide is isoelectronic with carbon dioxide , cyanate , nitrous oxide , nitronium ion and cyanogen fluoride NCF. Per valence bond theory, azide can be described ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Nitrite
The nitrite polyatomic ion, ion has the chemical formula . Nitrite (mostly sodium nitrite) is widely used throughout chemical and pharmaceutical industries. The nitrite anion is a pervasive intermediate in the nitrogen cycle in nature. The name nitrite also refers to organic compounds having the –ONO group, which are esters of nitrous acid. Production Sodium nitrite is made industrially by passing a mixture of nitrogen oxides into aqueous sodium hydroxide or sodium carbonate solution: : The product is purified by recrystallization. Alkali metal nitrites are thermally stable up to and beyond their melting point (441 °C for KNO2). Ammonium nitrite can be made from dinitrogen trioxide, N2O3, which is formally the anhydride of nitrous acid: :2 NH3 + H2O + N2O3 → 2 NH4NO2 Structure The nitrite ion has a symmetrical structure (C2v molecular point group, symmetry), with both N–O bonds having equal length and a bond angle of about 115°. In valence bond theory, it is des ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Carbon Suboxide
Carbon suboxide, or tricarbon dioxide, is an organic, oxygen-containing chemical compound with formula and structure . Its four cumulative double bonds make it a cumulene. It is one of the stable members of the series of linear oxocarbons , which also includes carbon dioxide () and pentacarbon dioxide (). Although if carefully purified it can exist at room temperature in the dark without decomposing, it will polymerize under certain conditions. The substance was discovered in 1873 by Benjamin Brodie by subjecting carbon monoxide to an electric current. He claimed that the product was part of a series of "oxycarbons" with formulas , namely , , , , …, and to have identified the last two; however, only is known. In 1891 Marcellin Berthelot observed that heating pure carbon monoxide at about 550 °C created small amounts of carbon dioxide but no trace of carbon, and assumed that a carbon-rich oxide was created instead, which he named "sub-oxide". He assumed it was the same ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Triphenylphosphine Oxide
Triphenylphosphine oxide (often abbreviated TPPO) is the organophosphorus compound with the formula OP(C6H5)3, also written as Ph3PO or PPh3O (Ph = phenyl, C6H5). This colourless crystalline compound is a common but potentially useful waste product in reactions involving triphenylphosphine. It is a popular reagent to induce the crystallization, crystallizing of chemical compounds. Structure and properties Ph3PO is a tetrahedral molecule related to POCl3. The oxygen center is relatively basic. The rigidity of the backbone and the basicity of the oxygen center make this species a popular agent to crystallize otherwise difficult to crystallize molecules. This trick is applicable to molecules that have acidic hydrogen atoms, e.g. phenols. Up to now, several modifications of Ph3PO have been found: For example, a monoclinic form crystalizes in the space group ''P''21/''c'' with Z = 4 and a = 15.066(1) Å, b = 9.037(2) Å, c = 11.296(3) Å, and β = 98.47(1)°. The orthorhombic modif ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |




nickel(II)-from-xtal-3D-balls.png)