|
Copperas Red
Iron(II) sulfate (British English: iron(II) sulphate) or ferrous sulfate denotes a range of salts with the formula Fe SO4·''x''H2O. These compounds exist most commonly as the heptahydrate (''x'' = 7) but several values for x are known. The hydrated form is used medically to treat iron deficiency, and also for industrial applications. Known since ancient times as copperas and as green vitriol (vitriol is an archaic name for sulfate), the blue-green heptahydrate (hydrate with 7 molecules of water) is the most common form of this material. All the iron(II) sulfates dissolve in water to give the same aquo complex e(H2O)6sup>2+, which has octahedral molecular geometry and is paramagnetic. The name copperas dates from times when the copper(II) sulfate was known as blue copperas, and perhaps in analogy, iron(II) and zinc sulfate were known respectively as green and white copperas. It is on the World Health Organization's List of Essential Medicines. In 2020, it was the 116t ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Ethanol
Ethanol (abbr. EtOH; also called ethyl alcohol, grain alcohol, drinking alcohol, or simply alcohol) is an organic compound. It is an Alcohol (chemistry), alcohol with the chemical formula . Its formula can be also written as or (an ethyl group linked to a hydroxyl group). Ethanol is a Volatility (chemistry), volatile, Combustibility and flammability, flammable, colorless liquid with a characteristic wine-like odor and pungent taste. It is a psychoactive recreational drug, the active ingredient in alcoholic drinks. Ethanol is naturally produced by the fermentation process of Carbohydrate, sugars by yeasts or via Petrochemistry, petrochemical processes such as ethylene hydration. It has medical applications as an antiseptic and disinfectant. It is used as a chemical solvent and in the Chemical synthesis, synthesis of organic compounds, and as a Alcohol fuel, fuel source. Ethanol also can be dehydrated to make ethylene, an important chemical feedstock. As of 2006, world produ ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Hydrate
In chemistry, a hydrate is a substance that contains water or its constituent elements. The chemical state of the water varies widely between different classes of hydrates, some of which were so labeled before their chemical structure was understood. Chemical nature Inorganic chemistry Hydrates are inorganic salts "containing water molecules combined in a definite ratio as an integral part of the crystal" that are either bound to a metal center or that have crystallized with the metal complex. Such hydrates are also said to contain ''water of crystallization'' or ''water of hydration''. If the water is heavy water in which the constituent hydrogen is the isotope deuterium, then the term ''deuterate'' may be used in place of ''hydrate''. A colorful example is cobalt(II) chloride, which turns from blue to red upon hydration, and can therefore be used as a water indicator. The notation "''hydrated compound''⋅''n''", where ''n'' is the number of water molecules per formula un ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Horticulture
Horticulture is the branch of agriculture that deals with the art, science, technology, and business of plant cultivation. It includes the cultivation of fruits, vegetables, nuts, seeds, herbs, sprouts, mushrooms, algae, flowers, seaweeds and non-food crops such as grass and ornamental trees and plants. It also includes plant conservation, landscape restoration, landscape and garden design, construction, and maintenance, and arboriculture, ornamental trees and lawns. The study and practice of horticulture have been traced back thousands of years. Horticulture contributed to the transition from nomadic human communities to sedentary, or semi-sedentary, horticultural communities.von Hagen, V.W. (1957) The Ancient Sun Kingdoms Of The Americas. Ohio: The World Publishing Company Horticulture is divided into several categories which focus on the cultivation and processing of different types of plants and food items for specific purposes. In order to conserve the science of horticultur ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Sulfuric Acid
Sulfuric acid (American spelling and the preferred IUPAC name) or sulphuric acid ( Commonwealth spelling), known in antiquity as oil of vitriol, is a mineral acid composed of the elements sulfur, oxygen and hydrogen, with the molecular formula . It is a colorless, odorless and viscous liquid that is miscible with water. Pure sulfuric acid does not exist naturally on Earth due to its strong affinity to water vapor; it is hygroscopic and readily absorbs water vapor from the air. Concentrated sulfuric acid is highly corrosive towards other materials, from rocks to metals, since it is an oxidant with powerful dehydrating properties. Phosphorus pentoxide is a notable exception in that it is not dehydrated by sulfuric acid, but to the contrary dehydrates sulfuric acid to sulfur trioxide. Upon addition of sulfuric acid to water, a considerable amount of heat is released; thus the reverse procedure of adding water to the acid should not be performed since the heat released may boi ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Iron Gall Ink
Iron gall ink (also known as common ink, standard ink, oak gall ink or iron gall nut ink) is a purple-black or brown-black ink made from iron salts and tannic acids from vegetable sources. It was the standard ink formulation used in Europe for the 1400-year period between the 5th and 19th centuries, remained in widespread use well into the 20th century, and is still sold today. Preparation and use The ink was traditionally prepared by adding some iron(II) sulfate ( Fe S O4) to a solution of tannic acid, but any iron ion donor can be used. The gallotannic acid was usually extracted from oak galls or galls of other trees, hence the name. Fermentation or hydrolysis of the extract releases glucose and gallic acid, which yields a darker purple-black ink, due to the formation of iron gallate. The fermented extract was combined with the iron(II) sulfate. After filtering, the resulting pale-grey solution had a binder added to it (most commonly gum arabic) and was used to write on pap ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Dye Fixative
A mordant or dye fixative is a substance used to set (i.e. bind) dyes on fabrics by forming a coordination complex with the dye, which then attaches to the fabric (or tissue). It may be used for dyeing fabrics or for intensifying stains in cell or tissue preparations. Although mordants are still used, especially by small batch dyers, it has been largely displaced in industry by directs.} The term mordant comes from the Latin ''mordere'', "to bite". In the past, it was thought that a mordant helped the dye bite onto the fiber so that it would hold fast during washing. A mordant is often a polyvalent metal ion, and one example is chromium (III). The resulting coordination complex of dye and ion is colloidal and can be either acidic or alkaline. Common dye mordants Mordants include tannic acid, oxalic acid, alum, chrome alum, sodium chloride, and certain salts of aluminium, chromium, copper, iron, iodine, potassium, sodium, tungsten, and tin. Iodine is often referred to ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Cement
A cement is a binder, a chemical substance used for construction that sets, hardens, and adheres to other materials to bind them together. Cement is seldom used on its own, but rather to bind sand and gravel ( aggregate) together. Cement mixed with fine aggregate produces mortar for masonry, or with sand and gravel, produces concrete. Concrete is the most widely used material in existence and is behind only water as the planet's most-consumed resource. Cements used in construction are usually inorganic, often lime or calcium silicate based, which can be characterized as hydraulic or the less common non-hydraulic, depending on the ability of the cement to set in the presence of water (see hydraulic and non-hydraulic lime plaster). Hydraulic cements (e.g., Portland cement) set and become adhesive through a chemical reaction between the dry ingredients and water. The chemical reaction results in mineral hydrates that are not very water-soluble and so are quite durable in wa ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
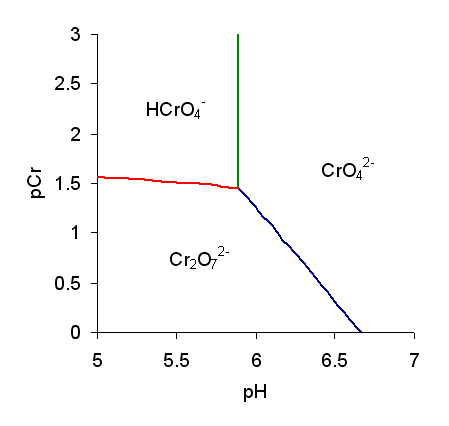
Chromate Ion
Chromate salts contain the chromate anion, . Dichromate salts contain the dichromate anion, . They are oxyanions of chromium in the +6 oxidation state and are moderately strong oxidizing agents. In an aqueous solution, chromate and dichromate ions can be interconvertible. Chemical properties Potassium-chromate-sample.jpg, potassium chromate Potassium-dichromate-sample.jpg, potassium dichromate Chromates react with hydrogen peroxide, giving products in which peroxide, , replaces one or more oxygen atoms. In acid solution the unstable blue peroxo complex Chromium(VI) oxide peroxide, CrO(O2)2, is formed; it is an uncharged covalent molecule, which may be extracted into ether. Addition of pyridine results in the formation of the more stable complex CrO(O2)2py. Acid–base properties In aqueous solution, chromate and dichromate anions exist in a chemical equilibrium. :2 + 2 H+ + H2O The predominance diagram shows that the position of the equilibrium depends on ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Reducing Agent
In chemistry, a reducing agent (also known as a reductant, reducer, or electron donor) is a chemical species that "donates" an electron to an (called the , , , or ). Examples of substances that are commonly reducing agents include the Earth metals, formic acid, oxalic acid, and sulfite compounds. In their pre-reaction states, reducers have extra electrons (that is, they are by themselves reduced) and oxidizers lack electrons (that is, they are by themselves oxidized). This is commonly expressed in terms of their oxidation states. An agent's oxidation state describes its degree of loss of electrons, where the higher the oxidation state then the fewer electrons it has. So initially, prior to the reaction, a reducing agent is typically in one of its lower possible oxidation states; its oxidation state increases during the reaction while that of the oxidizer decreases. Thus in a redox reaction, the agent whose oxidation state increases, that "loses/Electron donor, donates electrons ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
WHO Model List Of Essential Medicines
The WHO Model List of Essential Medicines (aka Essential Medicines List or EML), published by the World Health Organization (WHO), contains the medications considered to be most effective and safe to meet the most important needs in a health system. The list is frequently used by countries to help develop their own local lists of essential medicines. , more than 155 countries have created national lists of essential medicines based on the World Health Organization's model list. This includes both developed and developing countries. The list is divided into core items and complementary items. The core items are deemed to be the most cost-effective options for key health problems and are usable with little additional health care resources. The complementary items either require additional infrastructure such as specially trained health care providers or diagnostic equipment or have a lower cost–benefit ratio. About 25% of items are in the complementary list. Some medicatio ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Paramagnetic
Paramagnetism is a form of magnetism whereby some materials are weakly attracted by an externally applied magnetic field, and form internal, induced magnetic fields in the direction of the applied magnetic field. In contrast with this behavior, diamagnetic materials are repelled by magnetic fields and form induced magnetic fields in the direction opposite to that of the applied magnetic field. Paramagnetic materials include most chemical elements and some compounds; they have a relative magnetic permeability slightly greater than 1 (i.e., a small positive magnetic susceptibility) and hence are attracted to magnetic fields. The magnetic moment induced by the applied field is linear in the field strength and rather weak. It typically requires a sensitive analytical balance to detect the effect and modern measurements on paramagnetic materials are often conducted with a SQUID magnetometer. Paramagnetism is due to the presence of unpaired electrons in the material, so most atoms wi ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Octahedral Molecular Geometry
In chemistry, octahedral molecular geometry, also called square bipyramidal, describes the shape of compounds with six atoms or groups of atoms or ligands symmetrically arranged around a central atom, defining the vertices of an octahedron. The octahedron has eight faces, hence the prefix ''octa''. The octahedron is one of the Platonic solids, although octahedral molecules typically have an atom in their centre and no bonds between the ligand atoms. A perfect octahedron belongs to the point group Oh. Examples of octahedral compounds are sulfur hexafluoride SF6 and molybdenum hexacarbonyl Mo(CO)6. The term "octahedral" is used somewhat loosely by chemists, focusing on the geometry of the bonds to the central atom and not considering differences among the ligands themselves. For example, , which is not octahedral in the mathematical sense due to the orientation of the bonds, is referred to as octahedral. The concept of octahedral coordination geometry was developed by Alfred Wern ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |

_chloride.jpg)