|
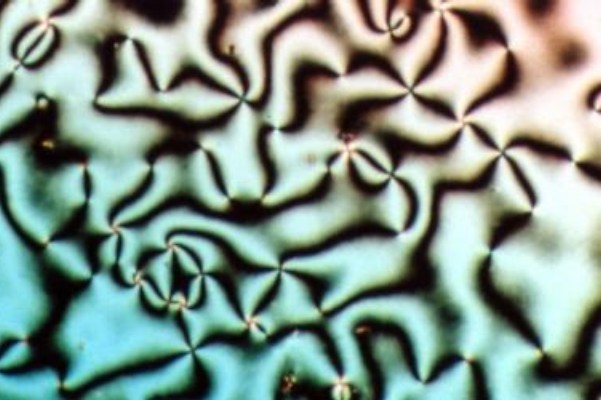
Columnar Phase
The columnar phase is a class of mesophases in which molecules assemble into cylindrical structures to act as mesogens. Originally, these kinds of liquid crystals were called discotic liquid crystals or bowlic liquid crystals because the columnar structures are composed of flat-shaped discotic or bowl-shaped molecules stacked one-dimensionally. Since recent findings provide a number of columnar liquid crystals consisting of non-discoid mesogens, it is more common now to classify this state of matter and compounds with these properties as columnar liquid crystals. Takuzo Aida and co-workers recently reported cyclic peptides that self-assemble into polar columnar organizations. These materials can be unidirectionally aligned over large areas by application of an external electric field. Classes Columnar liquid crystals are grouped by their structural order and the ways of packing of the columns. Nematic columnar liquid crystals have no long-range order and are less organized than o ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Mesophase
In chemistry and chemical physics, a mesophase or mesomorphic phase is a phase of matter intermediate between solid and liquid. Gelatin is a common example of a partially ordered structure in a mesophase. Further, biological structures such as the lipid bilayers of cell membranes are examples of mesophases. Mobile ions in mesophases are either orientationally or rotationally disordered while their centers are located at the ordered sites in the crystal structure. Mesophases with long-range positional order but no orientational order are plastic crystals, whereas those with long-range orientational order but only partial or no positional order are liquid crystals. Georges Friedel (1922) called attention to the "mesomorphic states of matter" in his scientific assessment of observations of the so-called liquid crystals. Conventionally a crystal is solid, and crystallization converts liquid to solid. The oxymoron of the liquid crystal is resolved through the notion of mesophases. The ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Mesogen
A mesogen is a compound that displays liquid crystal properties. Mesogens can be described as disordered solids or ordered liquids because they arise from a unique state of matter that exhibits both solid- and liquid-like properties called the liquid crystalline state. This liquid crystalline state (LC) is called the mesophase and occurs between the Crystal, crystalline solid (Cr) state and the isotropic liquid (Iso) state at distinct temperature ranges. The liquid crystal properties arise because mesogenic compounds are composed of rigid and flexible parts, which help characterize the order and mobility of its structure. The rigid components align mesogen Moiety (chemistry), moieties in one direction and have distinctive shapes that are typically found in the form of rod or disk shapes. The flexible segments provide mesogens with mobility because they are usually made up of alkyl chains, which hinder crystallization to a certain degree. The combination of rigid and flexible chain ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Liquid Crystal
Liquid crystal (LC) is a state of matter whose properties are between those of conventional liquids and those of solid crystals. For example, a liquid crystal can flow like a liquid, but its molecules may be oriented in a common direction as in a solid. There are many types of LC Phase (matter), phases, which can be distinguished by their Optics, optical properties (such as Texture (crystalline), textures). The contrasting textures arise due to molecules within one area of material ("domain") being oriented in the same direction but different areas having different orientations. An LC material may not always be in an LC state of matter (just as water may be ice or water vapour). Liquid crystals can be divided into three main types: thermotropic, lyotropic, and #Metallotropic liquid crystals, metallotropic. Thermotropic and lyotropic liquid crystals consist mostly of organic molecules, although a few minerals are also known. Thermotropic LCs exhibit a phase transition into the L ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
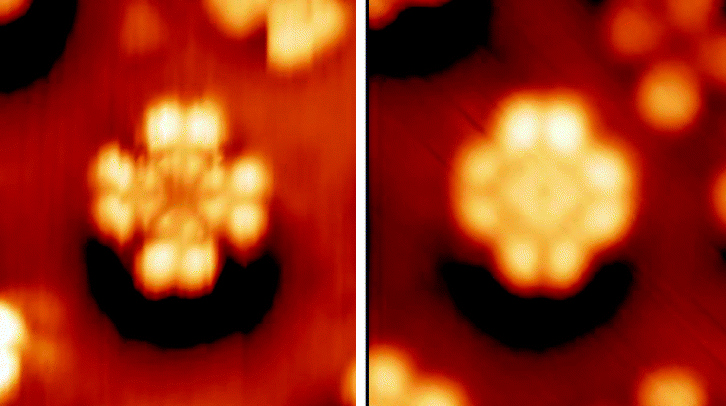
Discotic Liquid Crystal
Discotic liquid crystals are mesophases formed from disc-shaped molecules known as "discotic mesogens". These phases are often also referred to as columnar phases. Discotic mesogens are typically composed of an aromatic core surrounded by flexible alkyl chains. The aromatic cores allow charge transfer in the stacking direction through the π conjugate systems. The charge transfer allows the discotic liquid crystals to be electrically semiconductive along the stacking direction. Applications have been focusing on using these systems in photovoltaic devices, organic light emitting diodes (OLED), and molecular wires. Discotics have also been suggested for use in compensation films, for LCD displaysSivaramakrishna Chandrasekharwho first discovered these systems, did lots of important work on liquid crystals, and founded the International Liquid Crystal Society. He was Sir C V Raman's nephew, and brother of Pancharatnam of the geometric phase fame, and cousin of Subrahmanyam Chandrasek ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
State Of Matter
In physics, a state of matter is one of the distinct forms in which matter can exist. Four states of matter are observable in everyday life: solid, liquid, gas, and Plasma (physics), plasma. Different states are distinguished by the ways the component particles (atoms, molecules, ions and electrons) are arranged, and how they behave collectively. In a solid, the particles are tightly packed and held in fixed positions, giving the material a definite shape and volume. In a liquid, the particles remain close together but can move past one another, allowing the substance to maintain a fixed volume while adapting to the shape of its container. In a gas, the particles are far apart and move freely, allowing the substance to expand and fill both the shape and volume of its container. Plasma is similar to a gas, but it also contains charged particles (ions and free electrons) that move independently and respond to electric and magnetic fields. Beyond the classical states of matter, ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Nematic
Liquid crystal (LC) is a state of matter whose properties are between those of conventional liquids and those of solid crystals. For example, a liquid crystal can flow like a liquid, but its molecules may be oriented in a common direction as in a solid. There are many types of LC phases, which can be distinguished by their optical properties (such as textures). The contrasting textures arise due to molecules within one area of material ("domain") being oriented in the same direction but different areas having different orientations. An LC material may not always be in an LC state of matter (just as water may be ice or water vapour). Liquid crystals can be divided into three main types: thermotropic, lyotropic, and metallotropic. Thermotropic and lyotropic liquid crystals consist mostly of organic molecules, although a few minerals are also known. Thermotropic LCs exhibit a phase transition into the LC phase as temperature changes. Lyotropic LCs exhibit phase transitions a ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Sivaramakrishna Chandrasekhar
Sivaramakrishna Chandrasekhar FNA, FRS (6 August 1930 – 8 March 2004) was an Indian physicist who won the Royal Medal in 1994. He was the founder-president of the International Liquid Crystal Society. Chandrasekhar was born on 6 August 1930 at Kolkata, to (sister of C.V. Raman) and . He received his MSc degree in physics with first rank from Nagpur University in 1951. Subsequently, he joined the Raman Research Institute (RRI), Bangalore to work for his doctoral degree in physics under the guidance of his maternal uncle, C. V. Raman. The main topic of his research was related to optical rotatory dispersion measurements on several crystals. He received the D Sc degree from Nagpur University in 1954. Then he went to the Cavendish Laboratory on an 1851 Exhibition Scholarship and obtained a second doctorate degree from Cambridge University mainly for his work on the corrections for extinction in neutron and X-ray scattering from crystals. His subsequent postdoctoral work ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Benzene
Benzene is an Organic compound, organic chemical compound with the Chemical formula#Molecular formula, molecular formula C6H6. The benzene molecule is composed of six carbon atoms joined in a planar hexagonal Ring (chemistry), ring with one hydrogen atom attached to each. Because it contains only carbon and hydrogen atoms, benzene is classed as a hydrocarbon. Benzene is a natural constituent of petroleum and is one of the elementary petrochemicals. Due to the cyclic continuous pi bonds between the carbon atoms, benzene is classed as an aromatic hydrocarbon. Benzene is a colorless and highly Combustibility and flammability, flammable liquid with a sweet smell, and is partially responsible for the aroma of gasoline. It is used primarily as a Precursor (chemistry), precursor to the manufacture of chemicals with more complex structures, such as ethylbenzene and cumene, of which billions of kilograms are produced annually. Although benzene is a major Chemical industry, industrial che ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Alkane
In organic chemistry, an alkane, or paraffin (a historical trivial name that also has other meanings), is an acyclic saturated hydrocarbon. In other words, an alkane consists of hydrogen and carbon atoms arranged in a tree structure in which all the carbon–carbon bonds are single. Alkanes have the general chemical formula . The alkanes range in complexity from the simplest case of methane (), where ''n'' = 1 (sometimes called the parent molecule), to arbitrarily large and complex molecules, like hexacontane () or 4-methyl-5-(1-methylethyl) octane, an isomer of dodecane (). The International Union of Pure and Applied Chemistry (IUPAC) defines alkanes as "acyclic branched or unbranched hydrocarbons having the general formula , and therefore consisting entirely of hydrogen atoms and saturated carbon atoms". However, some sources use the term to denote ''any'' saturated hydrocarbon, including those that are either monocyclic (i.e. the cycloalkanes) or polycycl ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Triphenylene
Triphenylene is an organic compound with the formula (C6H4)3. It's a flat polycyclic aromatic hydrocarbon (PAH) that has a highly symmetric and planar structure consists of four fused benzene rings. Triphenylene has delocalized 18-''π''-electron systems based on a planar structure, corresponding to the symmetry group ''D''3h. It is more Resonance (chemistry), resonance stable than its isomers chrysene, Benz(a)anthracene, benz[''a'']anthracene, Benzo(c)phenanthrene, benzo[''c'']phenanthrene, and tetracene, hence resists hydrogenation. It is a light yellow powder, insoluble in water. Triphenylene serves as a fundamental building block in Discotic liquid crystal, discotic liquid crystals, where its planar, disc-like structure facilitates the formation of columnar mesophases, enabling applications in organic electronics. It's also being used as the base of covalent and metal organic frameworks. Discovery and First Synthesis Triphenylene was first separated by German Chemists H. Sc ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Porphyrin
Porphyrins ( ) are heterocyclic, macrocyclic, organic compounds, composed of four modified pyrrole subunits interconnected at their α carbon atoms via methine bridges (). In vertebrates, an essential member of the porphyrin group is heme, which is a component of hemoproteins, whose functions include carrying oxygen in the bloodstream. In plants, an essential porphyrin derivative is chlorophyll, which is involved in light harvesting and electron transfer in photosynthesis. The parent of porphyrins is porphine, a rare chemical compound of exclusively theoretical interest. Substituted porphines are called porphyrins. With a total of 26 π-electrons the porphyrin ring structure is a coordinated aromatic system. One result of the large conjugated system is that porphyrins absorb strongly in the visible region of the electromagnetic spectrum, i.e. they are deeply colored. The name "porphyrin" derives . Structure Porphyrin complexes consist of a square planar MN4 core. The p ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Phthalocyanine
Phthalocyanine () is a large, aromatic, macrocyclic, organic compound with the formula and is of theoretical or specialized interest in chemical dyes and photoelectricity. It is composed of four isoindole units linked by a ring of nitrogen atoms. = has a two-dimensional geometry and a ring system consisting of 18 π-electrons. The extensive delocalization of the π-electrons affords the molecule useful properties, lending itself to applications in dyes and pigments. Metal complexes derived from , the conjugate base of , are valuable in catalysis, organic solar cells, and photodynamic therapy. Properties Phthalocyanine and derived metal complexes (MPc) tend to aggregate and, thus, have low solubility in common solvents. Benzene at 40 °C dissolves less than a milligram of or CuPc per litre. and CuPc dissolve easily in sulfuric acid due to the protonation of the nitrogen atoms bridging the pyrrole rings. Many phthalocyanine compounds are, thermally, very s ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |