State Of Matter on:
[Wikipedia]
[Google]
[Amazon]
In

 In a solid, constituent particles (ions, atoms, or molecules) are closely packed together. The forces between particles are so strong that the particles cannot move freely but can only vibrate. As a result, a solid has a stable, definite shape, and a definite volume. Solids can only change their shape by an outside force, as when broken or cut.
In crystalline solids, the particles (atoms, molecules, or ions) are packed in a regularly ordered, repeating pattern. There are various different
In a solid, constituent particles (ions, atoms, or molecules) are closely packed together. The forces between particles are so strong that the particles cannot move freely but can only vibrate. As a result, a solid has a stable, definite shape, and a definite volume. Solids can only change their shape by an outside force, as when broken or cut.
In crystalline solids, the particles (atoms, molecules, or ions) are packed in a regularly ordered, repeating pattern. There are various different
 A gas is a compressible fluid. Not only will a gas conform to the shape of its container but it will also expand to fill the container.
In a gas, the molecules have enough
A gas is a compressible fluid. Not only will a gas conform to the shape of its container but it will also expand to fill the container.
In a gas, the molecules have enough
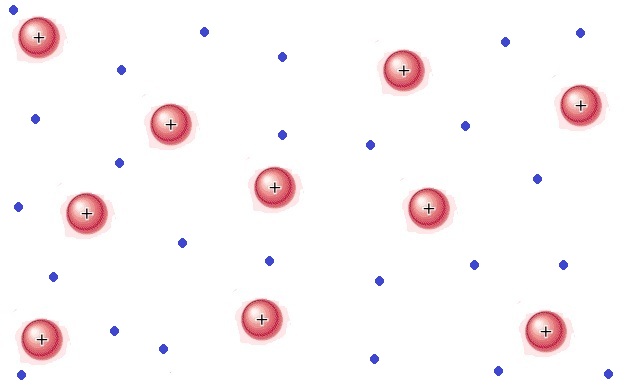 Like a gas, plasma does not have definite shape or volume. Unlike gases, plasmas are electrically conductive, produce magnetic fields and electric currents, and respond strongly to electromagnetic forces. Positively charged nuclei swim in a "sea" of freely-moving disassociated electrons, similar to the way such charges exist in conductive metal, where this electron "sea" allows matter in the plasma state to conduct electricity.
A gas is usually converted to a plasma in one of two ways, either from a huge voltage difference between two points, or by exposing it to extremely high temperatures. Heating matter to high temperatures causes electrons to leave the atoms, resulting in the presence of free electrons. This creates a so-called partially ionised plasma. At very high temperatures, such as those present in stars, it is assumed that essentially all electrons are "free", and that a very high-energy plasma is essentially bare nuclei swimming in a sea of electrons. This forms the so-called fully ionised plasma.
The plasma state is often misunderstood, and although not freely existing under normal conditions on Earth, it is quite commonly generated by either
Like a gas, plasma does not have definite shape or volume. Unlike gases, plasmas are electrically conductive, produce magnetic fields and electric currents, and respond strongly to electromagnetic forces. Positively charged nuclei swim in a "sea" of freely-moving disassociated electrons, similar to the way such charges exist in conductive metal, where this electron "sea" allows matter in the plasma state to conduct electricity.
A gas is usually converted to a plasma in one of two ways, either from a huge voltage difference between two points, or by exposing it to extremely high temperatures. Heating matter to high temperatures causes electrons to leave the atoms, resulting in the presence of free electrons. This creates a so-called partially ionised plasma. At very high temperatures, such as those present in stars, it is assumed that essentially all electrons are "free", and that a very high-energy plasma is essentially bare nuclei swimming in a sea of electrons. This forms the so-called fully ionised plasma.
The plasma state is often misunderstood, and although not freely existing under normal conditions on Earth, it is quite commonly generated by either
2005-06-22, MIT News: MIT physicists create new form of matter
Citat: "... They have become the first to create a new type of matter, a gas of atoms that shows high-temperature superfluidity."
* ttps://www.sciencedaily.com/releases/2004/01/040115074553.htm 2004-01-15, ScienceDaily: Probable Discovery Of A New, Supersolid, Phase Of MatterCitat: "...We apparently have observed, for the first time, a solid material with the characteristics of a superfluid...but because all its particles are in the identical quantum state, it remains a solid even though its component particles are continually flowing..."
2004-01-29, ScienceDaily: NIST/University Of Colorado Scientists Create New Form Of Matter: A Fermionic Condensate
Short videos demonstrating of States of Matter, solids, liquids and gases by Prof. J M Murrell, University of Sussex
{{DEFAULTSORT:State Of Matter Condensed matter physics Engineering thermodynamics
physics
Physics is the natural science that studies matter, its fundamental constituents, its motion and behavior through space and time, and the related entities of energy and force. "Physical science is that department of knowledge which r ...
, a state of matter is one of the distinct forms in which matter
In classical physics and general chemistry, matter is any substance that has mass and takes up space by having volume. All everyday objects that can be touched are ultimately composed of atoms, which are made up of interacting subatomic part ...
can exist. Four states of matter are observable in everyday life: solid
Solid is one of the four fundamental states of matter (the others being liquid, gas, and plasma). The molecules in a solid are closely packed together and contain the least amount of kinetic energy. A solid is characterized by structural ...
, liquid, gas
Gas is one of the four fundamental states of matter (the others being solid, liquid, and plasma).
A pure gas may be made up of individual atoms (e.g. a noble gas like neon), elemental molecules made from one type of atom (e.g. oxygen), or ...
, and plasma. Many intermediate states are known to exist, such as liquid crystal, and some states only exist under extreme conditions, such as Bose–Einstein condensate
In condensed matter physics, a Bose–Einstein condensate (BEC) is a state of matter that is typically formed when a gas of bosons at very low densities is cooled to temperatures very close to absolute zero (−273.15 °C or −459.6 ...
s (in extreme cold), neutron-degenerate matter
Degenerate matter is a highly dense state of fermionic matter in which the Pauli exclusion principle exerts significant pressure in addition to, or in lieu of, thermal pressure. The description applies to matter composed of electrons, protons, neu ...
(in extreme density), and quark–gluon plasma
Quark–gluon plasma (QGP) or quark soup is an interacting localized assembly of quarks and gluons at thermal (local kinetic) and (close to) chemical (abundance) equilibrium. The word ''plasma'' signals that free color charges are allowed. In a ...
(at extremely high energy). For a complete list of all exotic states of matter, see the list of states of matter
States of matter are distinguished by changes in the properties of matter associated with external factors like pressure and temperature. States are usually distinguished by a discontinuity in one of those properties—for example, raising the tem ...
.
Historically, the distinction is made based on qualitative differences in properties. Matter in the solid state maintains a fixed volume
Volume is a measure of occupied three-dimensional space. It is often quantified numerically using SI derived units (such as the cubic metre and litre) or by various imperial or US customary units (such as the gallon, quart, cubic inch). Th ...
(assuming no change in temperature or air pressure) and shape, with component particles ( atoms, molecules or ions
An ion () is an atom or molecule with a net electrical charge.
The charge of an electron is considered to be negative by convention and this charge is equal and opposite to the charge of a proton, which is considered to be positive by conven ...
) close together and fixed into place. Matter in the liquid state maintains a fixed volume (assuming no change in temperature or air pressure), but has a variable shape that adapts to fit its container. Its particles are still close together but move freely. Matter in the gaseous state has both variable volume and shape, adapting both to fit its container. Its particles are neither close together nor fixed in place. Matter in the plasma state has variable volume and shape, and contains neutral atoms as well as a significant number of ions and electrons
The electron ( or ) is a subatomic particle with a negative one elementary electric charge. Electrons belong to the first generation of the lepton particle family,
and are generally thought to be elementary particles because they have no ...
, both of which can move around freely.
The term "phase
Phase or phases may refer to:
Science
*State of matter, or phase, one of the distinct forms in which matter can exist
*Phase (matter), a region of space throughout which all physical properties are essentially uniform
* Phase space, a mathematic ...
" is sometimes used as a synonym for state of matter, but it is possible for a single compound to form different phases that are in the same state of matter. For example, ice
Ice is water frozen into a solid state, typically forming at or below temperatures of 0 degrees Celsius or Depending on the presence of impurities such as particles of soil or bubbles of air, it can appear transparent or a more or less opaqu ...
is the solid state of water, but there are multiple phases of ice with different crystal structure
In crystallography, crystal structure is a description of the ordered arrangement of atoms, ions or molecules in a crystal, crystalline material. Ordered structures occur from the intrinsic nature of the constituent particles to form symmetric pat ...
s, which are formed at different pressures and temperatures.
Four fundamental states

Solid
 In a solid, constituent particles (ions, atoms, or molecules) are closely packed together. The forces between particles are so strong that the particles cannot move freely but can only vibrate. As a result, a solid has a stable, definite shape, and a definite volume. Solids can only change their shape by an outside force, as when broken or cut.
In crystalline solids, the particles (atoms, molecules, or ions) are packed in a regularly ordered, repeating pattern. There are various different
In a solid, constituent particles (ions, atoms, or molecules) are closely packed together. The forces between particles are so strong that the particles cannot move freely but can only vibrate. As a result, a solid has a stable, definite shape, and a definite volume. Solids can only change their shape by an outside force, as when broken or cut.
In crystalline solids, the particles (atoms, molecules, or ions) are packed in a regularly ordered, repeating pattern. There are various different crystal structure
In crystallography, crystal structure is a description of the ordered arrangement of atoms, ions or molecules in a crystal, crystalline material. Ordered structures occur from the intrinsic nature of the constituent particles to form symmetric pat ...
s, and the same substance can have more than one structure (or solid phase). For example, iron
Iron () is a chemical element with symbol Fe (from la, ferrum) and atomic number 26. It is a metal that belongs to the first transition series and group 8 of the periodic table. It is, by mass, the most common element on Earth, right in f ...
has a body-centred cubic
In crystallography, the cubic (or isometric) crystal system is a crystal system where the unit cell is in the shape of a cube. This is one of the most common and simplest shapes found in crystals and minerals.
There are three main varieties of ...
structure at temperatures below , and a face-centred cubic
In crystallography, the cubic (or isometric) crystal system is a crystal system where the unit cell is in the shape of a cube. This is one of the most common and simplest shapes found in crystals and minerals.
There are three main varieties of ...
structure between 912 and . Ice
Ice is water frozen into a solid state, typically forming at or below temperatures of 0 degrees Celsius or Depending on the presence of impurities such as particles of soil or bubbles of air, it can appear transparent or a more or less opaqu ...
has fifteen known crystal structures, or fifteen solid phases, which exist at various temperatures and pressures.
Glass
Glass is a non-crystalline, often transparent, amorphous solid that has widespread practical, technological, and decorative use in, for example, window panes, tableware, and optics. Glass is most often formed by rapid cooling ( quenching ...
es and other non-crystalline, amorphous solid
In condensed matter physics and materials science, an amorphous solid (or non-crystalline solid, glassy solid) is a solid that lacks the long-range order that is characteristic of a crystal.
Etymology
The term comes from the Greek ''a'' ("wi ...
s without long-range order
In physics, the terms order and disorder designate the presence or absence of some symmetry or correlation in a many-particle system.
In condensed matter physics, systems typically are ordered at low temperatures; upon heating, they undergo one o ...
are not thermal equilibrium
Two physical systems are in thermal equilibrium if there is no net flow of thermal energy between them when they are connected by a path permeable to heat. Thermal equilibrium obeys the zeroth law of thermodynamics. A system is said to be i ...
ground states; therefore they are described below as nonclassical states of matter.
Solids can be transformed into liquids by melting, and liquids can be transformed into solids by freezing. Solids can also change directly into gases through the process of sublimation, and gases can likewise change directly into solids through deposition.
Liquid
A liquid is a nearly incompressible fluid that conforms to the shape of its container but retains a (nearly) constant volume independent of pressure. The volume is definite if thetemperature
Temperature is a physical quantity that expresses quantitatively the perceptions of hotness and coldness. Temperature is measurement, measured with a thermometer.
Thermometers are calibrated in various Conversion of units of temperature, temp ...
and pressure
Pressure (symbol: ''p'' or ''P'') is the force applied perpendicular to the surface of an object per unit area over which that force is distributed. Gauge pressure (also spelled ''gage'' pressure)The preferred spelling varies by country and e ...
are constant. When a solid is heated above its melting point
The melting point (or, rarely, liquefaction point) of a substance is the temperature at which it changes state from solid to liquid. At the melting point the solid and liquid phase exist in equilibrium. The melting point of a substance depen ...
, it becomes liquid, given that the pressure is higher than the triple point
In thermodynamics, the triple point of a substance is the temperature and pressure at which the three phases (gas, liquid, and solid) of that substance coexist in thermodynamic equilibrium.. It is that temperature and pressure at which the sub ...
of the substance. Intermolecular (or interatomic or interionic) forces are still important, but the molecules have enough energy to move relative to each other and the structure is mobile. This means that the shape of a liquid is not definite but is determined by its container. The volume is usually greater than that of the corresponding solid, the best known exception being water
Water (chemical formula ) is an inorganic, transparent, tasteless, odorless, and nearly colorless chemical substance, which is the main constituent of Earth's hydrosphere and the fluids of all known living organisms (in which it acts as a ...
, HO. The highest temperature at which a given liquid can exist is its critical temperature.
Gas
 A gas is a compressible fluid. Not only will a gas conform to the shape of its container but it will also expand to fill the container.
In a gas, the molecules have enough
A gas is a compressible fluid. Not only will a gas conform to the shape of its container but it will also expand to fill the container.
In a gas, the molecules have enough kinetic energy
In physics, the kinetic energy of an object is the energy that it possesses due to its motion.
It is defined as the work needed to accelerate a body of a given mass from rest to its stated velocity. Having gained this energy during its acc ...
so that the effect of intermolecular forces is small (or zero for an ideal gas
An ideal gas is a theoretical gas composed of many randomly moving point particles that are not subject to interparticle interactions. The ideal gas concept is useful because it obeys the ideal gas law, a simplified equation of state, and is a ...
), and the typical distance between neighboring molecules is much greater than the molecular size. A gas has no definite shape or volume, but occupies the entire container in which it is confined. A liquid may be converted to a gas by heating at constant pressure to the boiling point, or else by reducing the pressure at constant temperature.
At temperatures below its critical temperature, a gas is also called a vapor
In physics, a vapor (American English) or vapour (British English and Canadian English; see spelling differences) is a substance in the gas phase at a temperature lower than its critical temperature,R. H. Petrucci, W. S. Harwood, and F. G. Her ...
, and can be liquefied by compression alone without cooling. A vapor can exist in equilibrium with a liquid (or solid), in which case the gas pressure equals the vapor pressure
Vapor pressure (or vapour pressure in English-speaking countries other than the US; see spelling differences) or equilibrium vapor pressure is defined as the pressure exerted by a vapor in thermodynamic equilibrium with its condensed phas ...
of the liquid (or solid).
A supercritical fluid (SCF) is a gas whose temperature and pressure are above the critical temperature and critical pressure
In thermodynamics, a critical point (or critical state) is the end point of a phase equilibrium curve. The most prominent example is the liquid–vapor critical point, the end point of the pressure–temperature curve that designates conditions ...
respectively. In this state, the distinction between liquid and gas disappears. A supercritical fluid has the physical properties of a gas, but its high density confers solvent properties in some cases, which leads to useful applications. For example, supercritical carbon dioxide is used to extract caffeine
Caffeine is a central nervous system (CNS) stimulant of the methylxanthine class. It is mainly used recreationally as a cognitive enhancer, increasing alertness and attentional performance. Caffeine acts by blocking binding of adenosine to ...
in the manufacture of decaffeinated coffee.
Plasma
 Like a gas, plasma does not have definite shape or volume. Unlike gases, plasmas are electrically conductive, produce magnetic fields and electric currents, and respond strongly to electromagnetic forces. Positively charged nuclei swim in a "sea" of freely-moving disassociated electrons, similar to the way such charges exist in conductive metal, where this electron "sea" allows matter in the plasma state to conduct electricity.
A gas is usually converted to a plasma in one of two ways, either from a huge voltage difference between two points, or by exposing it to extremely high temperatures. Heating matter to high temperatures causes electrons to leave the atoms, resulting in the presence of free electrons. This creates a so-called partially ionised plasma. At very high temperatures, such as those present in stars, it is assumed that essentially all electrons are "free", and that a very high-energy plasma is essentially bare nuclei swimming in a sea of electrons. This forms the so-called fully ionised plasma.
The plasma state is often misunderstood, and although not freely existing under normal conditions on Earth, it is quite commonly generated by either
Like a gas, plasma does not have definite shape or volume. Unlike gases, plasmas are electrically conductive, produce magnetic fields and electric currents, and respond strongly to electromagnetic forces. Positively charged nuclei swim in a "sea" of freely-moving disassociated electrons, similar to the way such charges exist in conductive metal, where this electron "sea" allows matter in the plasma state to conduct electricity.
A gas is usually converted to a plasma in one of two ways, either from a huge voltage difference between two points, or by exposing it to extremely high temperatures. Heating matter to high temperatures causes electrons to leave the atoms, resulting in the presence of free electrons. This creates a so-called partially ionised plasma. At very high temperatures, such as those present in stars, it is assumed that essentially all electrons are "free", and that a very high-energy plasma is essentially bare nuclei swimming in a sea of electrons. This forms the so-called fully ionised plasma.
The plasma state is often misunderstood, and although not freely existing under normal conditions on Earth, it is quite commonly generated by either lightning
Lightning is a naturally occurring electrostatic discharge during which two electrically charged regions, both in the atmosphere or with one on the ground, temporarily neutralize themselves, causing the instantaneous release of an avera ...
, electric spark
An electric spark is an abrupt electrical discharge that occurs when a sufficiently high electric field creates an ionized, electrically conductive channel through a normally-insulating medium, often air or other gases or gas mixtures. Michael F ...
s, fluorescent lights
A fluorescent lamp, or fluorescent tube, is a low-pressure mercury-vapor gas-discharge lamp that uses fluorescence to produce visible light. An electric current in the gas excites mercury vapor, which produces short-wave ultraviolet lig ...
, neon lights or in plasma televisions. The Sun's corona
A corona ( coronas or coronae) is the outermost layer of a star's atmosphere. It consists of plasma.
The Sun's corona lies above the chromosphere and extends millions of kilometres into outer space. It is most easily seen during a total so ...
, some types of flame
A flame (from Latin '' flamma'') is the visible, gaseous part of a fire. It is caused by a highly exothermic chemical reaction taking place in a thin zone. When flames are hot enough to have ionized gaseous components of sufficient density they ...
, and stars are all examples of illuminated matter in the plasma state.
Phase transitions
A state of matter is also characterized byphase transitions
In chemistry, thermodynamics, and other related fields, a phase transition (or phase change) is the physical process of transition between one state of a medium and another. Commonly the term is used to refer to changes among the basic states of ...
. A phase transition indicates a change in structure and can be recognized by an abrupt change in properties. A distinct state of matter can be defined as any set of states distinguished from any other set of states by a phase transition
In chemistry, thermodynamics, and other related fields, a phase transition (or phase change) is the physical process of transition between one state of a medium and another. Commonly the term is used to refer to changes among the basic states o ...
. Water can be said to have several distinct solid states. The appearance of superconductivity is associated with a phase transition, so there are superconductive
Superconductivity is a set of physical properties observed in certain materials where electrical resistance vanishes and magnetic flux fields are expelled from the material. Any material exhibiting these properties is a superconductor. Unlike ...
states. Likewise, ferromagnetic states are demarcated by phase transitions and have distinctive properties.
When the change of state occurs in stages the intermediate steps are called mesophase In chemistry and chemical physics, a mesophase is a state of matter intermediate between liquid and solid. Gelatin is a common example of a partially ordered structure in a mesophase. Further, biological structures such as the lipid bilayers of cell ...
s. Such phases have been exploited by the introduction of liquid crystal technology.
The state or ''phase'' of a given set of matter can change depending on pressure
Pressure (symbol: ''p'' or ''P'') is the force applied perpendicular to the surface of an object per unit area over which that force is distributed. Gauge pressure (also spelled ''gage'' pressure)The preferred spelling varies by country and e ...
and temperature
Temperature is a physical quantity that expresses quantitatively the perceptions of hotness and coldness. Temperature is measurement, measured with a thermometer.
Thermometers are calibrated in various Conversion of units of temperature, temp ...
conditions, transitioning to other phases as these conditions change to favor their existence; for example, solid transitions to liquid with an increase in temperature. Near absolute zero, a substance exists as a solid
Solid is one of the four fundamental states of matter (the others being liquid, gas, and plasma). The molecules in a solid are closely packed together and contain the least amount of kinetic energy. A solid is characterized by structural ...
. As heat is added to this substance it melts into a liquid at its melting point
The melting point (or, rarely, liquefaction point) of a substance is the temperature at which it changes state from solid to liquid. At the melting point the solid and liquid phase exist in equilibrium. The melting point of a substance depen ...
, boils into a gas at its boiling point, and if heated high enough would enter a plasma state in which the electrons
The electron ( or ) is a subatomic particle with a negative one elementary electric charge. Electrons belong to the first generation of the lepton particle family,
and are generally thought to be elementary particles because they have no ...
are so energized that they leave their parent atoms.
Forms of matter that are not composed of molecules and are organized by different forces can also be considered different states of matter. Superfluids (like Fermionic condensate
A fermionic condensate or Fermi–Dirac condensate is a superfluid phase formed by fermionic particles at low temperatures. It is closely related to the Bose–Einstein condensate, a superfluid phase formed by bosonic atoms under similar cond ...
) and the quark–gluon plasma
Quark–gluon plasma (QGP) or quark soup is an interacting localized assembly of quarks and gluons at thermal (local kinetic) and (close to) chemical (abundance) equilibrium. The word ''plasma'' signals that free color charges are allowed. In a ...
are examples.
In a chemical equation, the state of matter of the chemicals may be shown as (s) for solid, (l) for liquid, and (g) for gas. An aqueous solution is denoted (aq). Matter in the plasma state is seldom used (if at all) in chemical equations, so there is no standard symbol to denote it. In the rare equations that plasma is used it is symbolized as (p).
Non-classical states
Glass
Glass
Glass is a non-crystalline, often transparent, amorphous solid that has widespread practical, technological, and decorative use in, for example, window panes, tableware, and optics. Glass is most often formed by rapid cooling ( quenching ...
is a non-crystalline or amorphous solid
In condensed matter physics and materials science, an amorphous solid (or non-crystalline solid, glassy solid) is a solid that lacks the long-range order that is characteristic of a crystal.
Etymology
The term comes from the Greek ''a'' ("wi ...
material that exhibits a glass transition when heated towards the liquid state. Glasses can be made of quite different classes of materials: inorganic networks (such as window glass, made of silicate plus additives), metallic alloys, ionic melts, aqueous solutions, molecular liquids, and polymers
A polymer (; Greek '' poly-'', "many" + ''-mer'', "part")
is a substance or material consisting of very large molecules called macromolecules, composed of many repeating subunits. Due to their broad spectrum of properties, both synthetic an ...
.
Thermodynamically, a glass is in a metastable state
In chemistry and physics, metastability denotes an intermediate energetic state within a dynamical system other than the system's state of least energy.
A ball resting in a hollow on a slope is a simple example of metastability. If the ball ...
with respect to its crystalline counterpart. The conversion rate, however, is practically zero.
Crystals with some degree of disorder
Aplastic crystal A plastic crystal is a crystal composed of weakly interacting molecules that possess some orientational or conformational degree of freedom. The name plastic crystal refers to the mechanical softness of such phases: they resemble waxes and are easil ...
is a molecular solid with long-range positional order but with constituent molecules retaining rotational freedom; in an orientational glass In solid-state physics, an orientational glass is a molecular solid in which crystalline long-range order coexists with quenched disorder in some rotational degree of freedom.
An orientational glass is either obtained by quenching a plastic cryst ...
this degree of freedom is frozen in a quenched disordered state.
Similarly, in a spin glass
In condensed matter physics, a spin glass is a magnetic state characterized by randomness, besides cooperative behavior in freezing of spins at a temperature called 'freezing temperature' ''Tf''. In ferromagnetic solids, component atoms' magne ...
magnetic disorder is frozen.
Liquid crystal states
Liquid crystal states have properties intermediate between mobile liquids and ordered solids. Generally, they are able to flow like a liquid, but exhibiting long-range order. For example, the nematic phase consists of long rod-like molecules such as para-azoxyanisole, which is nematic in the temperature range . In this state the molecules flow as in a liquid, but they all point in the same direction (within each domain) and cannot rotate freely. Like a crystalline solid, but unlike a liquid, liquid crystals react to polarized light. Other types of liquid crystals are described in the main article on these states. Several types have technological importance, for example, in liquid crystal displays.Magnetically ordered
Transition metal
In chemistry, a transition metal (or transition element) is a chemical element in the d-block of the periodic table (groups 3 to 12), though the elements of group 12 (and less often group 3) are sometimes excluded. They are the elements that ca ...
atoms often have magnetic moment
In electromagnetism, the magnetic moment is the magnetic strength and orientation of a magnet or other object that produces a magnetic field. Examples of objects that have magnetic moments include loops of electric current (such as electromagne ...
s due to the net spin of electrons that remain unpaired and do not form chemical bonds. In some solids the magnetic moments on different atoms are ordered and can form a ferromagnet, an antiferromagnet or a ferrimagnet.
In a ferromagnet
Ferromagnetism is a property of certain materials (such as iron) which results in a large observed magnetic permeability, and in many cases a large magnetic coercivity allowing the material to form a permanent magnet. Ferromagnetic materials ...
—for instance, solid iron
Iron () is a chemical element with symbol Fe (from la, ferrum) and atomic number 26. It is a metal that belongs to the first transition series and group 8 of the periodic table. It is, by mass, the most common element on Earth, right in f ...
—the magnetic moment on each atom is aligned in the same direction (within a magnetic domain
A magnetic domain is a region within a magnetic material in which the magnetization is in a uniform direction. This means that the individual magnetic moments of the atoms are aligned with one another and they point in the same direction. When c ...
). If the domains are also aligned, the solid is a permanent magnet
A magnet is a material or object that produces a magnetic field. This magnetic field is invisible but is responsible for the most notable property of a magnet: a force that pulls on other ferromagnetic materials, such as iron, steel, nicke ...
, which is magnetic even in the absence of an external magnetic field. The magnetization disappears when the magnet is heated to the Curie point
In physics and materials science, the Curie temperature (''T''C), or Curie point, is the temperature above which certain materials lose their permanent magnetic properties, which can (in most cases) be replaced by induced magnetism. The Cur ...
, which for iron is .
An antiferromagnet
In materials that exhibit antiferromagnetism, the magnetic moments of atoms or molecules, usually related to the spins of electrons, align in a regular pattern with neighboring spins (on different sublattices) pointing in opposite directions. ...
has two networks of equal and opposite magnetic moments, which cancel each other out so that the net magnetization is zero. For example, in nickel(II) oxide
Nickel is a chemical element with symbol Ni and atomic number 28. It is a silvery-white lustrous metal with a slight golden tinge. Nickel is a hard and ductile transition metal. Pure nickel is chemically reactive but large pieces are slow to r ...
(NiO), half the nickel atoms have moments aligned in one direction and half in the opposite direction.
In a ferrimagnet
A ferrimagnetic material is a material that has populations of atoms with opposing magnetic moments, as in antiferromagnetism, but these moments are unequal in magnitude so a spontaneous magnetization remains. This can for example occur when t ...
, the two networks of magnetic moments are opposite but unequal, so that cancellation is incomplete and there is a non-zero net magnetization. An example is magnetite
Magnetite is a mineral and one of the main iron ores, with the chemical formula Fe2+Fe3+2O4. It is one of the oxides of iron, and is ferrimagnetic; it is attracted to a magnet and can be magnetized to become a permanent magnet itself. With th ...
(FeO), which contains Fe and Fe ions with different magnetic moments.
A quantum spin liquid
In condensed matter physics, a quantum spin liquid is a phase of matter that can be formed by interacting quantum spins in certain magnetic materials. Quantum spin liquids (QSL) are generally characterized by their long-range quantum entanglem ...
(QSL) is a disordered state in a system of interacting quantum spins which preserves its disorder to very low temperatures, unlike other disordered states. It is not a liquid in physical sense, but a solid whose magnetic order is inherently disordered. The name "liquid" is due to an analogy with the molecular disorder in a conventional liquid. A QSL is neither a ferromagnet
Ferromagnetism is a property of certain materials (such as iron) which results in a large observed magnetic permeability, and in many cases a large magnetic coercivity allowing the material to form a permanent magnet. Ferromagnetic materials ...
, where magnetic domains are parallel, nor an antiferromagnet
In materials that exhibit antiferromagnetism, the magnetic moments of atoms or molecules, usually related to the spins of electrons, align in a regular pattern with neighboring spins (on different sublattices) pointing in opposite directions. ...
, where the magnetic domains are antiparallel; instead, the magnetic domains are randomly oriented. This can be realized e.g. by geometrically frustrated magnetic moments that cannot point uniformly parallel or antiparallel. When cooling down and settling to a state, the domain must "choose" an orientation, but if the possible states are similar in energy, one will be chosen randomly. Consequently, despite strong short-range order, there is no long-range magnetic order.
Microphase-separated

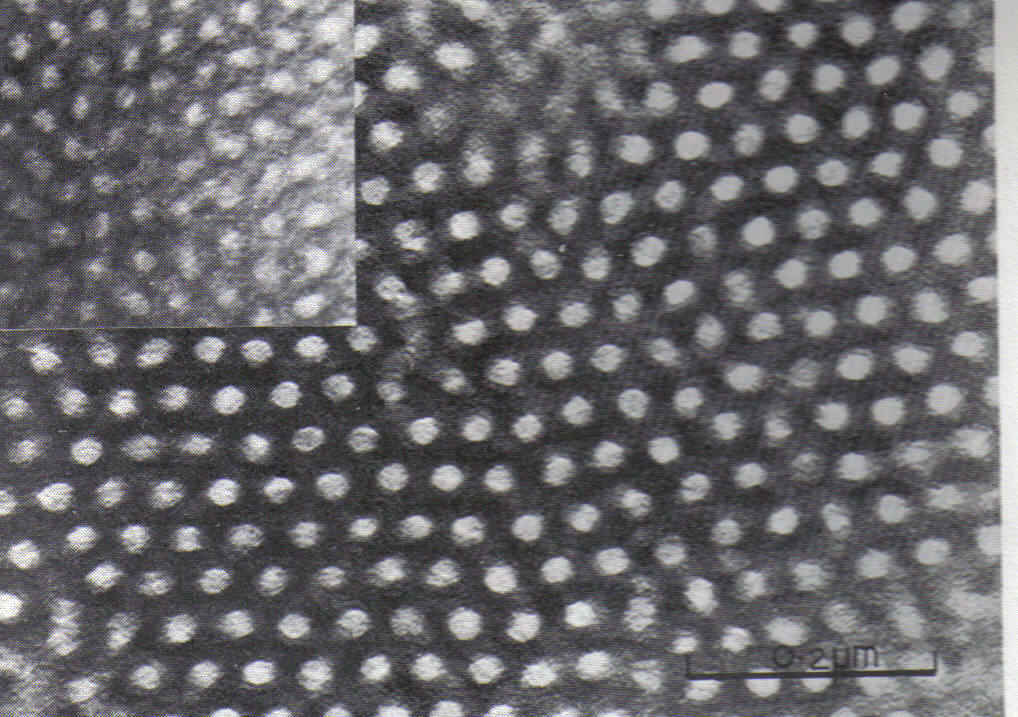
Copolymers
In polymer chemistry, a copolymer is a polymer derived from more than one species of monomer. The polymerization of monomers into copolymers is called copolymerization. Copolymers obtained from the copolymerization of two monomer species are some ...
can undergo microphase separation to form a diverse array of periodic nanostructures, as shown in the example of the styrene-butadiene-styrene block copolymer shown at right. Microphase separation can be understood by analogy to the phase separation between oil
An oil is any nonpolar chemical substance that is composed primarily of hydrocarbons and is hydrophobic (does not mix with water) & lipophilic (mixes with other oils). Oils are usually flammable and surface active. Most oils are unsaturated ...
and water. Due to chemical incompatibility between the blocks, block copolymers undergo a similar phase separation. However, because the blocks are covalently bonded
A covalent bond is a chemical bond that involves the sharing of electrons to form electron pairs between atoms. These electron pairs are known as shared pairs or bonding pairs. The stable balance of attractive and repulsive forces between atoms ...
to each other, they cannot demix macroscopically as water and oil can, and so instead the blocks form nanometre-sized structures. Depending on the relative lengths of each block and the overall block topology of the polymer, many morphologies can be obtained, each its own phase of matter.
Ionic liquid
An ionic liquid (IL) is a salt in the liquid state. In some contexts, the term has been restricted to salts whose melting point is below a specific temperature, such as . While ordinary liquids such as water and gasoline are predominantly made of ...
s also display microphase separation. The anion and cation are not necessarily compatible and would demix otherwise, but electric charge attraction prevents them from separating. Their anions and cations appear to diffuse within compartmentalized layers or micelles instead of freely as in a uniform liquid.
Low-temperature states
Superconductor
Superconductors are materials which have zeroelectrical resistivity
Electrical resistivity (also called specific electrical resistance or volume resistivity) is a fundamental property of a material that measures how strongly it resists electric current. A low resistivity indicates a material that readily allows ...
, and therefore perfect conductivity. This is a distinct physical state which exists at low temperature, and the resistivity increases discontinuously to a finite value at a sharply-defined transition temperature for each superconductor.
A superconductor also excludes all magnetic fields from its interior, a phenomenon known as the Meissner effect
The Meissner effect (or Meissner–Ochsenfeld effect) is the expulsion of a magnetic field from a superconductor during its transition to the superconducting state when it is cooled below the critical temperature. This expulsion will repel a ne ...
or perfect diamagnetism
Diamagnetic materials are repelled by a magnetic field; an applied magnetic field creates an induced magnetic field in them in the opposite direction, causing a repulsive force. In contrast, paramagnetic and ferromagnetic materials are attracte ...
. Superconducting magnets are used as electromagnets in magnetic resonance imaging machines.
The phenomenon of superconductivity was discovered in 1911, and for 75 years was only known in some metals and metallic alloys at temperatures below 30 K. In 1986 so-called high-temperature superconductivity
High-temperature superconductors (abbreviated high-c or HTS) are defined as materials that behave as superconductors at temperatures above , the boiling point of liquid nitrogen. The adjective "high temperature" is only in respect to previ ...
was discovered in certain ceramic
A ceramic is any of the various hard, brittle, heat-resistant and corrosion-resistant materials made by shaping and then firing an inorganic, nonmetallic material, such as clay, at a high temperature. Common examples are earthenware, porcelain ...
oxides, and has now been observed in temperatures as high as 164 K.
Superfluid
Close to absolute zero, some liquids form a second liquid state described as superfluid because it has zeroviscosity
The viscosity of a fluid is a measure of its resistance to deformation at a given rate. For liquids, it corresponds to the informal concept of "thickness": for example, syrup has a higher viscosity than water.
Viscosity quantifies the inte ...
(or infinite fluidity; i.e., flowing without friction). This was discovered in 1937 for helium
Helium (from el, ἥλιος, helios, lit=sun) is a chemical element with the symbol He and atomic number 2. It is a colorless, odorless, tasteless, non-toxic, inert, monatomic gas and the first in the noble gas group in the periodic table. ...
, which forms a superfluid below the lambda temperature of . In this state it will attempt to "climb" out of its container. It also has infinite thermal conductivity
The thermal conductivity of a material is a measure of its ability to conduct heat. It is commonly denoted by k, \lambda, or \kappa.
Heat transfer occurs at a lower rate in materials of low thermal conductivity than in materials of high thermal ...
so that no temperature gradient can form in a superfluid. Placing a superfluid in a spinning container will result in quantized vortices.
These properties are explained by the theory that the common isotope helium-4
Helium-4 () is a stable isotope of the element helium. It is by far the more abundant of the two naturally occurring isotopes of helium, making up about 99.99986% of the helium on Earth. Its nucleus is identical to an alpha particle, and consis ...
forms a Bose–Einstein condensate
In condensed matter physics, a Bose–Einstein condensate (BEC) is a state of matter that is typically formed when a gas of bosons at very low densities is cooled to temperatures very close to absolute zero (−273.15 °C or −459.6 ...
(see next section) in the superfluid state. More recently, Fermionic condensate
A fermionic condensate or Fermi–Dirac condensate is a superfluid phase formed by fermionic particles at low temperatures. It is closely related to the Bose–Einstein condensate, a superfluid phase formed by bosonic atoms under similar cond ...
superfluids have been formed at even lower temperatures by the rare isotope helium-3 and by lithium-6
Naturally occurring lithium (3Li) is composed of two stable isotopes, lithium-6 and lithium-7, with the latter being far more abundant on Earth. Both of the natural isotopes have an unexpectedly low nuclear binding energy per nucleon ( for ...
.
Bose–Einstein condensate
In 1924,Albert Einstein
Albert Einstein ( ; ; 14 March 1879 – 18 April 1955) was a German-born theoretical physicist, widely acknowledged to be one of the greatest and most influential physicists of all time. Einstein is best known for developing the theory ...
and Satyendra Nath Bose
Satyendra Nath Bose (; 1 January 1894 – 4 February 1974) was a Bengali mathematician and physicist specializing in theoretical physics. He is best known for his work on quantum mechanics in the early 1920s, in developing the foundation for ...
predicted the "Bose–Einstein condensate" (BEC), sometimes referred to as the fifth state of matter. In a BEC, matter stops behaving as independent particles, and collapses into a single quantum state that can be described with a single, uniform wavefunction.
In the gas phase, the Bose–Einstein condensate remained an unverified theoretical prediction for many years. In 1995, the research groups of Eric Cornell
Eric Allin Cornell (born December 19, 1961) is an American physicist who, along with Carl E. Wieman, was able to synthesize the first Bose–Einstein condensate in 1995. For their efforts, Cornell, Wieman, and Wolfgang Ketterle shared the Nobel ...
and Carl Wieman, of JILA
JILA, formerly known as the Joint Institute for Laboratory Astrophysics, is a physical science research institute in the United States. JILA is located on the University of Colorado Boulder campus. JILA was founded in 1962 as a joint institute ...
at the University of Colorado at Boulder
The University of Colorado Boulder (CU Boulder, CU, or Colorado) is a public research university in Boulder, Colorado. Founded in 1876, five months before Colorado became a state, it is the flagship university of the University of Colorado sys ...
, produced the first such condensate experimentally. A Bose–Einstein condensate is "colder" than a solid. It may occur when atoms have very similar (or the same) quantum levels, at temperatures very close to absolute zero, .
Fermionic condensate
A ''fermionic condensate'' is similar to the Bose–Einstein condensate but composed of fermions. ThePauli exclusion principle
In quantum mechanics, the Pauli exclusion principle states that two or more identical particles with half-integer spins (i.e. fermions) cannot occupy the same quantum state within a quantum system simultaneously. This principle was formulat ...
prevents fermions from entering the same quantum state, but a pair of fermions can behave as a boson, and multiple such pairs can then enter the same quantum state without restriction.
Rydberg molecule
One of themetastable state
In chemistry and physics, metastability denotes an intermediate energetic state within a dynamical system other than the system's state of least energy.
A ball resting in a hollow on a slope is a simple example of metastability. If the ball ...
s of strongly non-ideal plasma are condensates of excited atoms, called Rydberg matter
Rydberg matter is an exotic phase (matter), phase of matter formed by Rydberg atoms; it was predicted around 1980 by É. A. Manykin, M. I. Ozhovan and P. P. Poluéktov. It has been formed from various elements like caesium, potassium, hydrogen and ...
. These atoms can also turn into ion
An ion () is an atom or molecule with a net electrical charge.
The charge of an electron is considered to be negative by convention and this charge is equal and opposite to the charge of a proton, which is considered to be positive by conve ...
s and electron
The electron ( or ) is a subatomic particle with a negative one elementary electric charge. Electrons belong to the first generation of the lepton particle family,
and are generally thought to be elementary particles because they have no ...
s if they reach a certain temperature. In April 2009, ''Nature
Nature, in the broadest sense, is the physics, physical world or universe. "Nature" can refer to the phenomenon, phenomena of the physical world, and also to life in general. The study of nature is a large, if not the only, part of science. ...
'' reported the creation of Rydberg molecules from a Rydberg atom and a ground state atom, confirming that such a state of matter could exist. The experiment was performed using ultracold rubidium atoms.
Quantum Hall state
A ''quantum Hall state'' gives rise to quantizedHall voltage
The Hall effect is the production of a voltage difference (the Hall voltage) across an electrical conductor that is transverse to an electric current in the conductor and to an applied magnetic field perpendicular to the current. It was discove ...
measured in the direction perpendicular to the current flow. A '' quantum spin Hall state'' is a theoretical phase that may pave the way for the development of electronic devices that dissipate less energy and generate less heat. This is a derivation of the Quantum Hall state of matter.
Photonic matter
Photonic matter is a phenomenon wherephoton
A photon () is an elementary particle that is a quantum of the electromagnetic field, including electromagnetic radiation such as light and radio waves, and the force carrier for the electromagnetic force. Photons are massless, so they a ...
s interacting with a gas develop apparent mass, and can interact with each other, even forming photonic "molecules". The source of mass is the gas, which is massive. This is in contrast to photons moving in empty space, which have no rest mass
The invariant mass, rest mass, intrinsic mass, proper mass, or in the case of bound systems simply mass, is the portion of the total mass of an object or system of objects that is independent of the overall motion of the system. More precisely, i ...
, and cannot interact.
Dropleton
A "quantum fog" of electrons and holes that flow around each other and even ripple like a liquid, rather than existing as discrete pairs.High-energy states
Degenerate matter
Under extremely high pressure, as in the cores of dead stars, ordinary matter undergoes a transition to a series of exotic states of matter collectively known asdegenerate matter
Degenerate matter is a highly dense state of fermionic matter in which the Pauli exclusion principle exerts significant pressure in addition to, or in lieu of, thermal pressure. The description applies to matter composed of electrons, protons, n ...
, which are supported mainly by quantum mechanical effects. In physics, "degenerate" refers to two states that have the same energy and are thus interchangeable. Degenerate matter is supported by the Pauli exclusion principle
In quantum mechanics, the Pauli exclusion principle states that two or more identical particles with half-integer spins (i.e. fermions) cannot occupy the same quantum state within a quantum system simultaneously. This principle was formulat ...
, which prevents two fermionic particles from occupying the same quantum state. Unlike regular plasma, degenerate plasma expands little when heated, because there are simply no momentum states left. Consequently, degenerate stars collapse into very high densities. More massive degenerate stars are smaller, because the gravitational force increases, but pressure does not increase proportionally.
Electron-degenerate matter
Degenerate matter is a highly dense state of fermionic matter in which the Pauli exclusion principle exerts significant pressure in addition to, or in lieu of, thermal pressure. The description applies to matter composed of electrons, protons, neu ...
is found inside white dwarf
A white dwarf is a stellar core remnant composed mostly of electron-degenerate matter. A white dwarf is very dense: its mass is comparable to the Sun's, while its volume is comparable to the Earth's. A white dwarf's faint luminosity comes ...
stars. Electrons remain bound to atoms but are able to transfer to adjacent atoms. Neutron-degenerate matter
Degenerate matter is a highly dense state of fermionic matter in which the Pauli exclusion principle exerts significant pressure in addition to, or in lieu of, thermal pressure. The description applies to matter composed of electrons, protons, neu ...
is found in neutron star
A neutron star is the collapsed core of a massive supergiant star, which had a total mass of between 10 and 25 solar masses, possibly more if the star was especially metal-rich. Except for black holes and some hypothetical objects (e.g. w ...
s. Vast gravitational pressure compresses atoms so strongly that the electrons are forced to combine with protons via inverse beta-decay, resulting in a superdense conglomeration of neutrons. Normally free neutron
The neutron is a subatomic particle, symbol or , which has a neutral (not positive or negative) charge, and a mass slightly greater than that of a proton. Protons and neutrons constitute the nuclei of atoms. Since protons and neutrons beh ...
s outside an atomic nucleus will decay
Decay may refer to:
Science and technology
* Bit decay, in computing
* Software decay, in computing
* Distance decay, in geography
* Decay time (fall time), in electronics
Biology
* Decomposition of organic matter
* Tooth decay (dental caries ...
with a half life of approximately 10 minutes, but in a neutron star, the decay is overtaken by inverse decay. Cold degenerate matter is also present in planets such as Jupiter
Jupiter is the fifth planet from the Sun and the largest in the Solar System. It is a gas giant with a mass more than two and a half times that of all the other planets in the Solar System combined, but slightly less than one-thousandth t ...
and in the even more massive brown dwarf
Brown dwarfs (also called failed stars) are substellar objects that are not massive enough to sustain nuclear fusion of ordinary hydrogen ( 1H) into helium in their cores, unlike a main-sequence star. Instead, they have a mass between the most ...
s, which are expected to have a core with metallic hydrogen
Metallic hydrogen is a phase of hydrogen in which it behaves like an electrical conductor. This phase was predicted in 1935 on theoretical grounds by Eugene Wigner and Hillard Bell Huntington.
At high pressure and temperatures, metallic hydroge ...
. Because of the degeneracy, more massive brown dwarfs are not significantly larger. In metals, the electrons can be modeled as a degenerate gas moving in a lattice of non-degenerate positive ions.
Quark matter
In regular cold matter, quarks, fundamental particles of nuclear matter, are confined by thestrong force
The strong interaction or strong force is a fundamental interaction that confines quarks into proton, neutron, and other hadron particles. The strong interaction also binds neutrons and protons to create atomic nuclei, where it is called the ...
into hadron
In particle physics, a hadron (; grc, ἁδρός, hadrós; "stout, thick") is a composite subatomic particle made of two or more quarks held together by the strong interaction. They are analogous to molecules that are held together by the e ...
s that consist of 2–4 quarks, such as protons and neutrons. Quark matter or quantum chromodynamical (QCD) matter is a group of phases where the strong force is overcome and quarks are deconfined and free to move. Quark matter phases occur at extremely high densities or temperatures, and there are no known ways to produce them in equilibrium in the laboratory; in ordinary conditions, any quark matter formed immediately undergoes radioactive decay.
Strange matter
Strange matter (or strange quark matter) is quark matter containing strange quarks. In nature, strange matter is hypothesized to occur in the core of neutron stars, or, more speculatively, as isolated droplets that may vary in size from femtome ...
is a type of quark matter
Quark matter or QCD matter (quantum chromodynamic) refers to any of a number of hypothetical phases of matter whose degrees of freedom include quarks and gluons, of which the prominent example is quark-gluon plasma. Several series of conferences ...
that is suspected to exist inside some neutron stars close to the Tolman–Oppenheimer–Volkoff limit The Tolman–Oppenheimer–Volkoff limit (or TOV limit) is an upper bound to the mass of cold, nonrotating neutron stars, analogous to the Chandrasekhar limit for white dwarf stars. If the mass of the said star reaches the limit it will collapse to ...
(approximately 2–3 solar masses), although there is no direct evidence of its existence. In strange matter, part of the energy available manifests as strange quarks, a heavier analogue of the common down quark
The down quark or d quark (symbol: d) is the second-lightest of all quarks, a type of elementary particle, and a major constituent of matter. Together with the up quark, it forms the neutrons (one up quark, two down quarks) and protons (two up ...
. It may be stable at lower energy states once formed, although this is not known.
Quark–gluon plasma
Quark–gluon plasma (QGP) or quark soup is an interacting localized assembly of quarks and gluons at thermal (local kinetic) and (close to) chemical (abundance) equilibrium. The word ''plasma'' signals that free color charges are allowed. In a ...
is a very high-temperature phase in which quarks become free and able to move independently, rather than being perpetually bound into particles, in a sea of gluons, subatomic particles that transmit the strong force
The strong interaction or strong force is a fundamental interaction that confines quarks into proton, neutron, and other hadron particles. The strong interaction also binds neutrons and protons to create atomic nuclei, where it is called the ...
that binds quarks together. This is analogous to the liberation of electrons from atoms in a plasma. This state is briefly attainable in extremely high-energy heavy ion collisions in particle accelerator
A particle accelerator is a machine that uses electromagnetic fields to propel charged particles to very high speeds and energies, and to contain them in well-defined beams.
Large accelerators are used for fundamental research in particle ...
s, and allows scientists to observe the properties of individual quarks, and not just theorize. Quark–gluon plasma was discovered at CERN
The European Organization for Nuclear Research, known as CERN (; ; ), is an intergovernmental organization that operates the largest particle physics laboratory in the world. Established in 1954, it is based in a northwestern suburb of Gene ...
in 2000. Unlike plasma, which flows like a gas, interactions within QGP are strong and it flows like a liquid.
At high densities but relatively low temperatures, quarks are theorized to form a quark liquid whose nature is presently unknown. It forms a distinct color-flavor locked (CFL) phase at even higher densities. This phase is superconductive
Superconductivity is a set of physical properties observed in certain materials where electrical resistance vanishes and magnetic flux fields are expelled from the material. Any material exhibiting these properties is a superconductor. Unlike ...
for color charge. These phases may occur in neutron star
A neutron star is the collapsed core of a massive supergiant star, which had a total mass of between 10 and 25 solar masses, possibly more if the star was especially metal-rich. Except for black holes and some hypothetical objects (e.g. w ...
s but they are presently theoretical.
Color-glass condensate
Color-glass condensate is a type of matter theorized to exist in atomic nuclei traveling near the speed of light. According to Einstein's theory of relativity, a high-energy nucleus appears length contracted, or compressed, along its direction of motion. As a result, the gluons inside the nucleus appear to a stationary observer as a "gluonic wall" traveling near the speed of light. At very high energies, the density of the gluons in this wall is seen to increase greatly. Unlike the quark–gluon plasma produced in the collision of such walls, the color-glass condensate describes the walls themselves, and is an intrinsic property of the particles that can only be observed under high-energy conditions such as those at RHIC and possibly at the Large Hadron Collider as well.Very high energy states
Various theories predict new states of matter at very high energies. An unknown state has created thebaryon asymmetry
In physical cosmology, the baryon asymmetry problem, also known as the matter asymmetry problem or the matter–antimatter asymmetry problem, is the observed imbalance in baryonic matter (the type of matter experienced in everyday life) and antib ...
in the universe, but little is known about it. In string theory, a Hagedorn temperature
The Hagedorn temperature, ''T''H, is the temperature in theoretical physics where hadronic matter (i.e. ordinary matter) is no longer stable, and must either "evaporate" or convert into quark matter; as such, it can be thought of as the "boiling p ...
is predicted for superstrings at about 1030 K, where superstrings are copiously produced. At Planck temperature (1032 K), gravity becomes a significant force between individual particles. No current theory can describe these states and they cannot be produced with any foreseeable experiment. However, these states are important in cosmology
Cosmology () is a branch of physics and metaphysics dealing with the nature of the universe. The term ''cosmology'' was first used in English in 1656 in Thomas Blount's ''Glossographia'', and in 1731 taken up in Latin by German philosopher ...
because the universe may have passed through these states in the Big Bang.
The gravitational singularity
A gravitational singularity, spacetime singularity or simply singularity is a condition in which gravity is so intense that spacetime itself breaks down catastrophically. As such, a singularity is by definition no longer part of the regular sp ...
predicted by general relativity
General relativity, also known as the general theory of relativity and Einstein's theory of gravity, is the geometric theory of gravitation published by Albert Einstein in 1915 and is the current description of gravitation in modern physics ...
to exist at the center of a black hole is ''not'' a phase of matter; it is not a material object at all (although the mass-energy of matter contributed to its creation) but rather a property of spacetime
In physics, spacetime is a mathematical model that combines the three dimensions of space and one dimension of time into a single four-dimensional manifold. Spacetime diagrams can be used to visualize relativistic effects, such as why differ ...
. Because spacetime breaks down there, the singularity should not be thought of as a localized structure, but as a global, topological feature of spacetime. It has been argued that elementary particles are fundamentally not material, either, but are localized properties of spacetime. In quantum gravity, singularities may in fact mark transitions to a new phase of matter.
Other proposed states
Supersolid
A supersolid is a spatially ordered material (that is, a solid or crystal) with superfluid properties. Similar to a superfluid, a supersolid is able to move without friction but retains a rigid shape. Although a supersolid is a solid, it exhibits so many characteristic properties different from other solids that many argue it is another state of matter.String-net liquid
In a string-net liquid, atoms have apparently unstable arrangement, like a liquid, but are still consistent in overall pattern, like a solid. When in a normal solid state, the atoms of matter align themselves in a grid pattern, so that the spin of any electron is the opposite of the spin of all electrons touching it. But in a string-net liquid, atoms are arranged in some pattern that requires some electrons to have neighbors with the same spin. This gives rise to curious properties, as well as supporting some unusual proposals about the fundamental conditions of the universe itself.Superglass
A superglass is a phase of matter characterized, at the same time, bysuperfluid
Superfluidity is the characteristic property of a fluid with zero viscosity which therefore flows without any loss of kinetic energy. When stirred, a superfluid forms vortices that continue to rotate indefinitely. Superfluidity occurs in two ...
ity and a frozen amorphous structure.
Arbitrary definition
Although multiple attempts have been made to create a unified account, ultimately the definitions of what states of matter exist and the point at which states change are arbitrary. Some authors have suggested that states of matter are better thought of as aspectrum
A spectrum (plural ''spectra'' or ''spectrums'') is a condition that is not limited to a specific set of values but can vary, without gaps, across a continuum. The word was first used scientifically in optics to describe the rainbow of colors i ...
between a solid and plasma instead of being rigidly defined.
See also
* Hidden states of matter *Classical element
Classical elements typically refer to earth, water, air, fire, and (later) aether which were proposed to explain the nature and complexity of all matter in terms of simpler substances. Ancient cultures in Greece, Tibet, and India had simil ...
* Condensed matter physics
* Cooling curve
A cooling curve is a line graph that represents the change of phase of matter, typically from a gas to a solid or a liquid to a solid. The independent variable (X-axis) is time and the dependent variable (Y-axis) is temperature.Garland, Nibler, ...
* Phase (matter)
In the physical sciences, a phase is a region of space (a thermodynamic system), throughout which all physical properties of a material are essentially uniform. Examples of physical properties include density, index of refraction, magneti ...
* Supercooling
Supercooling, also known as undercooling, is the process of lowering the temperature of a liquid or a gas below its melting point without it becoming a solid. It achieves this in the absence of a seed crystal or nucleus around which a crystal ...
* Superheating
Notes and references
External links
2005-06-22, MIT News: MIT physicists create new form of matter
Citat: "... They have become the first to create a new type of matter, a gas of atoms that shows high-temperature superfluidity."
* ttps://www.sciencedaily.com/releases/2004/01/040115074553.htm 2004-01-15, ScienceDaily: Probable Discovery Of A New, Supersolid, Phase Of MatterCitat: "...We apparently have observed, for the first time, a solid material with the characteristics of a superfluid...but because all its particles are in the identical quantum state, it remains a solid even though its component particles are continually flowing..."
2004-01-29, ScienceDaily: NIST/University Of Colorado Scientists Create New Form Of Matter: A Fermionic Condensate
Short videos demonstrating of States of Matter, solids, liquids and gases by Prof. J M Murrell, University of Sussex
{{DEFAULTSORT:State Of Matter Condensed matter physics Engineering thermodynamics