|
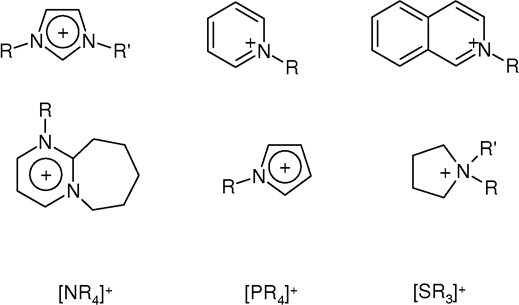
Ionic Liquid
An ionic liquid (IL) is a salt in the liquid state. In some contexts, the term has been restricted to salts whose melting point is below a specific temperature, such as . While ordinary liquids such as water and gasoline are predominantly made of electrically neutral molecules, ionic liquids are largely made of ions. These substances are variously called liquid electrolytes, ionic melts, ionic fluids, fused salts, liquid salts, or ionic glasses. Ionic liquids have many potential applications. They are powerful solvents and can be used as electrolytes. Salts that are liquid at near-ambient temperature are important for electric battery applications, and have been considered as sealants due to their very low vapor pressure. Any salt that melts without decomposing or vaporizing usually yields an ionic liquid. Sodium chloride (NaCl), for example, melts at into a liquid that consists largely of sodium cations () and chloride anions (). Conversely, when an ionic liquid is cooled, ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Bmim
1-Butyl-3-methylimidazolium hexafluorophosphate, also known as BMIM-PF6, is a viscous, colourless, hydrophobe, hydrophobic and non-water-soluble ionic liquid with a melting point of -8 °C. Together with 1-butyl-3-methylimidazolium tetrafluoroborate, BMIM-BF4, it is one of the most widely studied ionic liquids. It is known to very slowly decompose in the presence of water. Preparation BMIM-PF6 is commercially available. It may be obtained in two steps: BMIM-Cl is synthesized by alkylating 1-methylimidazole with 1-chlorobutane. A metathesis reaction with potassium hexafluorophosphate gives the desired compound; the tetrafluoroborate may be prepared by analogously using potassium tetrafluoroborate. : See also * 1-Butyl-3-methylimidazolium tetrachloroferrate References Further reading * {{DEFAULTSORT:Butyl-3-methylimidazolium hexafluorophosphate, 1- Hexafluorophosphates Imidazolium compounds Ionic liquids ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Sodium Chloride
Sodium chloride , commonly known as salt (although sea salt also contains other chemical salts), is an ionic compound with the chemical formula NaCl, representing a 1:1 ratio of sodium and chloride ions. With molar masses of 22.99 and 35.45 g/mol respectively, 100 g of NaCl contains 39.34 g Na and 60.66 g Cl. Sodium chloride is the salt most responsible for the salinity of seawater and of the extracellular fluid of many multicellular organisms. In its edible form, salt (also known as ''table salt'') is commonly used as a condiment and food preservative. Large quantities of sodium chloride are used in many industrial processes, and it is a major source of sodium and chlorine compounds used as feedstocks for further chemical syntheses. Another major application of sodium chloride is de-icing of roadways in sub-freezing weather. Uses In addition to the familiar domestic uses of salt, more dominant applications of the approximately 250 million tonnes per year production (2008 ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Nylanderia Fulva
''Nylanderia'' is a large genus of ants in the subfamily Formicinae. The genus has a nearly cosmopolitan distribution with species inhabiting a wide array of habitats in almost all geographic regions. ''Nylanderia'', currently containing over 110 species, is an ecologically important genus, with some species reported as being invasive. The ants are small to medium in size and range in color from pale yellow to black. Taxonomy The genus was first described as a subgenus of ''Prenolepis'' by Emery (1906), a status he revised a couple of years later when he placed it as subgenus of ''Paratrechina'' (Emery, 1925). Wheeler (1936) raised ''Nylanderia'' to genus, where it remained until Brown (1973) provisionally placed it as a junior synonym of ''Paratrechina'', a status which was later confirmed by Trager (1984). ''Nylanderia'' was finally revived from synonymy and restored at the rank of genus by LaPolla, Brady & Shattuck (2010). Until 2010, most ''Nylanderia'' species were place ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Trans
Trans- is a Latin prefix meaning "across", "beyond", or "on the other side of". Used alone, trans may refer to: Arts, entertainment, and media * Trans (festival), a former festival in Belfast, Northern Ireland, United Kingdom * ''Trans'' (film), a 1998 American film * Trans Corp, an Indonesian business unit of CT Corp in the fields of media, lifestyle, and entertainment ** Trans Media, a media subsidiary of Trans Corp *** Trans TV, an Indonesian television network *** Trans7, an Indonesian television network Literature * '' Trans: Gender and Race in an Age of Unsettled Identities'', a 2016 book by Rogers Brubaker * '' Trans: When Ideology Meets Reality'', a 2021 book by Helen Joyce Music * ''Trans'' (album), by Neil Young * ''Trans'' (Stockhausen), a 1971 orchestral composition Places * Trans, Mayenne, France, a commune * Trans, Switzerland, a village Science and technology * Trans effect in inorganic chemistry, the increased lability of ligands that are trans to certain o ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Deep Eutectic Solvent
Deep eutectic solvents or DESs are solutions of Lewis or Brønsted acids and bases which form a eutectic mixture. Deep eutectic solvents are highly tunable through varying the structure or relative ratio of parent components and thus have a wide variety of potential applications including catalytic, separation, and electrochemical processes. The parent components of deep eutectic solvents engage in a complex hydrogen bonding network which results in significant freezing point depression as compared to the parent compounds. The extent of freezing point depression observed in DESs is well illustrated by a mixture of choline chloride and urea in a 1:2 mole ratio. Choline chloride and urea are both solids at room temperature with melting points of 302°C (decomposition point) and 133°C respectively, yet the combination of the two in a 1:2 molar ratio forms a liquid with a freezing point of 12°C. DESs share similar properties to ionic liquids such as tunability and lack of flammability ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |