|
Chukanovite
Chukanovite is an iron(II) hydroxide-carbonate mineral with the ideal chemical formula Fe+22(CO3)(OH)2. It is a member of the rosasite mineral group and crystalizes in the monoclinic crystal system. Upon initial crystallization, it is typically pale green to colorless, but it takes on a brownish green hue after being altered at the surface. As a weathering product of meteoritic iron, chukanovite is a relatively uncommon mineral on Earth, having only been discovered in the year 2000. However, it is commonly formed artificially as a corrosion byproduct through the manufacturing of sand-deposited carbon steel. Occurrence Chukanovite was first discovered in weathered cavities of a meteorite which fell near the small village of Dronino, 350 km southeast of Moscow, Russia, but the mineral has since been found elsewhere in cavities of other iron-rich meteorites. It occurs primarily in association with goethite, akaganeite, hematite, hibbingite, reevesite, honessite, and kamacite, though ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Carbonate
A carbonate is a salt of carbonic acid (H2CO3), characterized by the presence of the carbonate ion, a polyatomic ion with the formula . The word ''carbonate'' may also refer to a carbonate ester, an organic compound containing the carbonate group C(=O)(O–)2. The term is also used as a verb, to describe carbonation: the process of raising the concentrations of carbonate and bicarbonate ions in water to produce carbonated water and other carbonated beverageseither by the addition of carbon dioxide gas under pressure or by dissolving carbonate or bicarbonate salts into the water. In geology and mineralogy, the term "carbonate" can refer both to carbonate minerals and carbonate rock (which is made of chiefly carbonate minerals), and both are dominated by the carbonate ion, . Carbonate minerals are extremely varied and ubiquitous in chemically precipitated sedimentary rock. The most common are calcite or calcium carbonate, CaCO3, the chief constituent of limestone (as well a ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Hematite
Hematite (), also spelled as haematite, is a common iron oxide compound with the formula, Fe2O3 and is widely found in rocks and soils. Hematite crystals belong to the rhombohedral lattice system which is designated the alpha polymorph of . It has the same crystal structure as corundum () and ilmenite (). With this it forms a complete solid solution at temperatures above . Hematite naturally occurs in black to steel or silver-gray, brown to reddish-brown, or red colors. It is mined as an important ore mineral of iron. It is electrically conductive. Hematite varieties include ''kidney ore'', ''martite'' (pseudomorphs after magnetite), ''iron rose'' and ''specularite'' (specular hematite). While these forms vary, they all have a rust-red streak. Hematite is not only harder than pure iron, but also much more brittle. Maghemite is a polymorph of hematite (γ-) with the same chemical formula, but with a spinel structure like magnetite. Large deposits of hematite are found in ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
List Of Minerals
This is a list of minerals for which there are articles on Wikipedia. Minerals are distinguished by various chemical and physical properties. Differences in chemical composition and crystal structure distinguish the various ''species''. Within a mineral species there may be variation in physical properties or minor amounts of impurities that are recognized by mineralogists or wider society as a mineral ''variety''. Mineral variety names are listed after the valid minerals for each letter. For a more complete listing of all mineral names, see List of minerals recognized by the International Mineralogical Association. A :Varieties that are not valid species: *Adamantine spar (variety of corundum) *Agate (variety of chalcedony and quartz) *Alabaster (variety of gypsum) *Alexandrite (variety of chrysoberyl) *Allingite (synonym of amber) *Alum *Amazonite (variety of microcline) *Amethyst (purple variety of quartz) *Ametrine (variety of quartz) *Ammolite (organic; also a gems ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Powder Diffraction
Powder diffraction is a scientific technique using X-ray, neutron, or electron diffraction on powder or microcrystalline samples for structural characterization of materials. An instrument dedicated to performing such powder measurements is called a powder diffractometer. Powder diffraction stands in contrast to single crystal diffraction techniques, which work best with a single, well-ordered crystal. Explanation A diffractometer produces electromagnetic radiation (waves) with known wavelength and frequency, which is determined by their source. The source is often x-rays, because they are the only kind of energy with the optimal wavelength for inter-atomic-scale diffraction. However, electrons and neutrons are also common sources, with their frequency determined by their de Broglie wavelength. When these waves reach the sample, the incoming beam is either reflected off the surface, or can enter the lattice and be diffracted by the atoms present in the sample. If the atoms are ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Empirical Formula
In chemistry, the empirical formula of a chemical compound is the simplest whole number ratio of atoms present in a compound. A simple example of this concept is that the empirical formula of sulfur monoxide, or SO, would simply be SO, as is the empirical formula of disulfur dioxide, S2O2. Thus, sulfur monoxide and disulfur dioxide, both compounds of sulfur and oxygen, have the same empirical formula. However, their molecular formulas, which express the number of atoms in each molecule of a chemical compound, are not the same. An empirical formula makes no mention of the arrangement or number of atoms. It is standard for many ionic compounds, like calcium chloride (CaCl2), and for macromolecules, such as silicon dioxide (SiO2). The molecular formula, on the other hand, shows the number of each type of atom in a molecule. The structural formula shows the arrangement of the molecule. It is also possible for different types of compounds to have equal empirical formulas. Sampl ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Magnesium
Magnesium is a chemical element with the symbol Mg and atomic number 12. It is a shiny gray metal having a low density, low melting point and high chemical reactivity. Like the other alkaline earth metals (group 2 of the periodic table) it occurs naturally only in combination with other elements and it almost always has an oxidation state of +2. It reacts readily with air to form a thin passivation coating of magnesium oxide that inhibits further corrosion of the metal. The free metal burns with a brilliant-white light. The metal is obtained mainly by electrolysis of magnesium salts obtained from brine. It is less dense than aluminium and is used primarily as a component in strong and lightweight alloys that contain aluminium. In the cosmos, magnesium is produced in large, aging stars by the sequential addition of three helium nuclei to a carbon nucleus. When such stars explode as supernovas, much of the magnesium is expelled into the interstellar medium where it ma ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Nickel
Nickel is a chemical element with symbol Ni and atomic number 28. It is a silvery-white lustrous metal with a slight golden tinge. Nickel is a hard and ductile transition metal. Pure nickel is chemically reactive but large pieces are slow to react with air under standard conditions because a passivation layer of nickel oxide forms on the surface that prevents further corrosion. Even so, pure native nickel is found in Earth's crust only in tiny amounts, usually in ultramafic rocks, and in the interiors of larger nickel–iron meteorites that were not exposed to oxygen when outside Earth's atmosphere. Meteoric nickel is found in combination with iron, a reflection of the origin of those elements as major end products of supernova nucleosynthesis. An iron–nickel mixture is thought to compose Earth's outer and inner cores. Use of nickel (as natural meteoric nickel–iron alloy) has been traced as far back as 3500 BCE. Nickel was first isolated and classified as an e ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Calcite
Calcite is a Carbonate minerals, carbonate mineral and the most stable Polymorphism (materials science), polymorph of calcium carbonate (CaCO3). It is a very common mineral, particularly as a component of limestone. Calcite defines hardness 3 on the Mohs scale of mineral hardness, based on Scratch hardness, scratch hardness comparison. Large calcite crystals are used in optical equipment, and limestone composed mostly of calcite has numerous uses. Other polymorphs of calcium carbonate are the minerals aragonite and vaterite. Aragonite will change to calcite over timescales of days or less at temperatures exceeding 300 °C, and vaterite is even less stable. Etymology Calcite is derived from the German ''Calcit'', a term from the 19th century that came from the Latin word for Lime (material), lime, ''calx'' (genitive calcis) with the suffix "-ite" used to name minerals. It is thus etymologically related to chalk. When applied by archaeology, archaeologists and stone trade pr ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
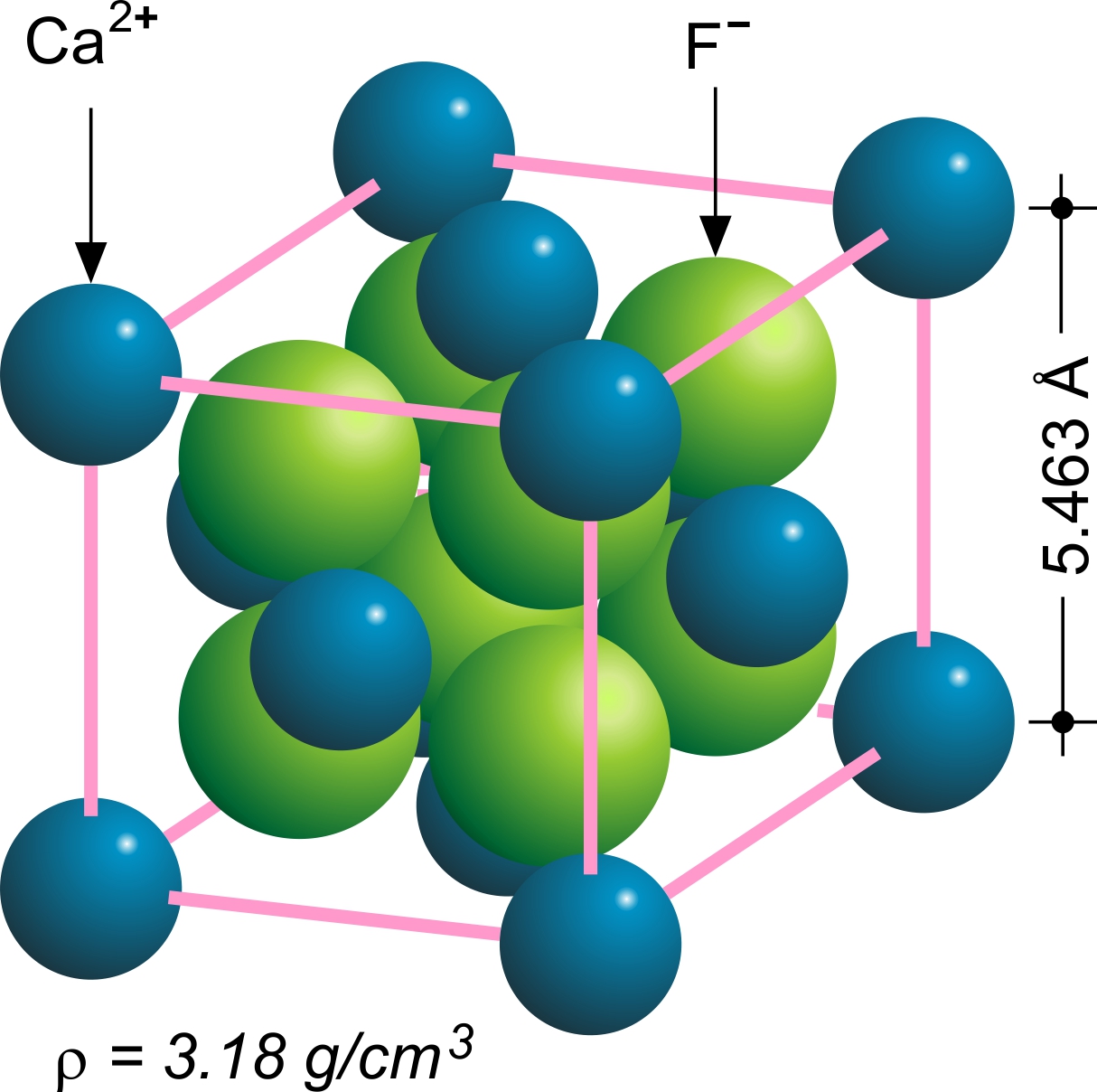
Fluorite
Fluorite (also called fluorspar) is the mineral form of calcium fluoride, CaF2. It belongs to the halide minerals. It crystallizes in isometric cubic habit, although octahedral and more complex isometric forms are not uncommon. The Mohs scale of mineral hardness, based on scratch hardness comparison, defines value 4 as fluorite. Pure fluorite is colourless and transparent, both in visible and ultraviolet light, but impurities usually make it a colorful mineral and the stone has ornamental and lapidary uses. Industrially, fluorite is used as a flux for smelting, and in the production of certain glasses and enamels. The purest grades of fluorite are a source of fluoride for hydrofluoric acid manufacture, which is the intermediate source of most fluorine-containing fine chemicals. Optically clear transparent fluorite lenses have low dispersion, so lenses made from it exhibit less chromatic aberration, making them valuable in microscopes and telescopes. Fluorite optics are also ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Mohs Scale Of Mineral Hardness
The Mohs scale of mineral hardness () is a qualitative ordinal scale, from 1 to 10, characterizing scratch resistance of various minerals through the ability of harder material to scratch softer material. The scale was introduced in 1812 by the German geologist and mineralogist Friedrich Mohs, in his book ''"Versuch einer Elementar-Methode zur naturhistorischen Bestimmung und Erkennung der Fossilien"''; it is one of several definitions of hardness in materials science, some of which are more quantitative. The method of comparing hardness by observing which minerals can scratch others is of great antiquity, having been mentioned by Theophrastus in his treatise ''On Stones'', , followed by Pliny the Elder in his ''Naturalis Historia'', . The Mohs scale is useful for identification of minerals in the field, but is not an accurate predictor of how well materials endure in an industrial setting – ''toughness''. Minerals The Mohs scale of mineral hardness is based on the ability ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Spherulites
In petrology, spherulites () are small, rounded bodies that commonly occur in vitreous igneous rocks. They are often visible in specimens of obsidian, pitchstone, and rhyolite as globules about the size of millet seed or rice grain, with a duller luster than the surrounding glassy base of the rock, and when they are examined with a lens they prove to have a radiate fibrous structure. Structure Under the microscope the spherulites are of circular outline and are composed of thin divergent fibers that are crystalline as verified with polarized light. Between crossed Nicols, a black cross appears in the spherulite; its axes are usually perpendicular to one another and parallel to the crosshairs; as the microscope stage is rotated the cross remains steady; between the black arms there are four bright sectors. This shows that the spherulite consists of radiate, doubly refracting fibers that have a straight extinction; the arms of the black cross correspond to those fibers that ar ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Carbon Dioxide
Carbon dioxide (chemical formula ) is a chemical compound made up of molecules that each have one carbon atom covalently double bonded to two oxygen atoms. It is found in the gas state at room temperature. In the air, carbon dioxide is transparent to visible light but absorbs infrared radiation, acting as a greenhouse gas. It is a trace gas in Earth's atmosphere at 421 parts per million (ppm), or about 0.04% by volume (as of May 2022), having risen from pre-industrial levels of 280 ppm. Burning fossil fuels is the primary cause of these increased CO2 concentrations and also the primary cause of climate change.IPCC (2022Summary for policy makersiClimate Change 2022: Mitigation of Climate Change. Contribution of Working Group III to the Sixth Assessment Report of the Intergovernmental Panel on Climate Change Cambridge University Press, Cambridge, United Kingdom and New York, NY, USA Carbon dioxide is soluble in water and is found in groundwater, lakes, ice caps, ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |