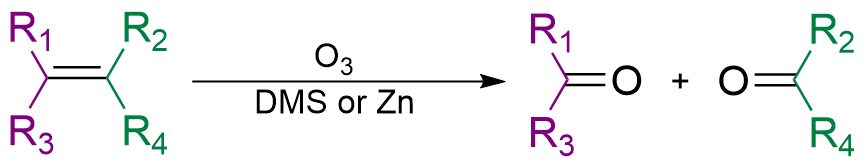|
Cefroxadine
Cefroxadine (INN, trade names Oraspor and Cefthan-DS) is a cephalosporin antibiotic. It is structurally related to cefalexin, and both drugs share a similar spectrum of activity. It is available in Italy. Synthesis Cefroxadine can be prepared by several routes, including one in which the enol is methylated with diazomethane as a key step. A rather more involved route starts with comparatively readily available phenoxymethylpenicillin sulfoxide benzhydryl ester (1). This undergoes fragmentation when treated with benzothiazole-2-thiol to give 2. Ozonolysis (reductive work-up) cleaves the olefinic linkage and the unsymmetrical disulfide moiety is converted to a tosyl thioester (3). The enol moiety is methylated with diazomethane, the six-membered ring is closed by reaction with 1,5-diazabicyclo .4.0ndec-5-ene (DBU), and the ester protection is removed with trifluoroacetic acid to give 4. The amide side chain is removed by the usual PCl5/dimethylaniline ''N'',''N''-Dimethylanil ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Cefroxadine Synthesis
Cefroxadine ( INN, trade names Oraspor and Cefthan-DS) is a cephalosporin antibiotic. It is structurally related to cefalexin, and both drugs share a similar spectrum of activity. It is available in Italy. Synthesis Cefroxadine can be prepared by several routes, including one in which the enol is methylated with diazomethane as a key step. A rather more involved route starts with comparatively readily available phenoxymethylpenicillin sulfoxide benzhydryl ester (1). This undergoes fragmentation when treated with benzothiazole-2-thiol to give 2. Ozonolysis (reductive work-up) cleaves the olefinic linkage and the unsymmetrical disulfide moiety is converted to a tosyl thioester (3). The enol moiety is methylated with diazomethane, the six-membered ring is closed by reaction with 1,5-diazabicyclo .4.0ndec-5-ene (DBU), and the ester protection is removed with trifluoroacetic acid to give 4. The amide side chain is removed by the usual PCl5/dimethylaniline ''N'',''N''-Dimethyl ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Cephalosporin
The cephalosporins (sg. ) are a class of β-lactam antibiotics originally derived from the fungus '' Acremonium'', which was previously known as ''Cephalosporium''. Together with cephamycins, they constitute a subgroup of β-lactam antibiotics called cephems. Cephalosporins were discovered in 1945, and first sold in 1964. Discovery The aerobic mold which yielded cephalosporin C was found in the sea near a sewage outfall in Su Siccu, by Cagliari harbour in Sardinia, by the Italian pharmacologist Giuseppe Brotzu in July 1945. Structure Cephalosporin contains a 6-membered dihydrothiazine ring. Substitutions at position 3 generally affect pharmacology; substitutions at position 7 affect antibacterial activity, but these cases are not always true. Medical uses Cephalosporins can be indicated for the prophylaxis and treatment of infections caused by bacteria susceptible to this particular form of antibiotic. First-generation cephalosporins are active predominantly ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Kidney
The kidneys are two reddish-brown bean-shaped organs found in vertebrates. They are located on the left and right in the retroperitoneal space, and in adult humans are about in length. They receive blood from the paired renal arteries; blood exits into the paired renal veins. Each kidney is attached to a ureter, a tube that carries excreted urine to the bladder. The kidney participates in the control of the volume of various body fluids, fluid osmolality, acid–base balance, various electrolyte concentrations, and removal of toxins. Filtration occurs in the glomerulus: one-fifth of the blood volume that enters the kidneys is filtered. Examples of substances reabsorbed are solute-free water, sodium, bicarbonate, glucose, and amino acids. Examples of substances secreted are hydrogen, ammonium, potassium and uric acid. The nephron is the structural and functional unit of the kidney. Each adult human kidney contains around 1 million nephrons, while a mouse kidney ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Cefalexin
Cefalexin, also spelled cephalexin, is an antibiotic that can treat a number of bacterial infections. It kills gram-positive and some gram-negative bacteria by disrupting the growth of the bacterial cell wall. Cefalexin is a beta-lactam antibiotic within the class of first-generation cephalosporins. It works similarly to other agents within this class, including intravenous cefazolin, but can be taken by mouth. Cefalexin can treat certain bacterial infections, including those of the middle ear, bone and joint, skin, and urinary tract. It may also be used for certain types of pneumonia and strep throat and to prevent bacterial endocarditis. Cefalexin is not effective against infections caused by methicillin-resistant ''Staphylococcus aureus'' (MRSA), most '' Enterococcus'', or '' Pseudomonas''. Like other antibiotics, cefalexin cannot treat viral infections, such as the flu, common cold or acute bronchitis. Cefalexin can be used in those who have mild or moderat ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Antimicrobial Pharmacodynamics
Antimicrobial pharmacodynamics is the relationship between the concentration of an antibiotic and its ability to inhibit vital processes of endo- or ectoparasites and microbial organisms.C.H. Nightingale, T. Murakawa, P.G. Ambrose (2002) Antimicrobial Pharmacodynamics in Theory and Clinical Practice Informa Health Care This branch of pharmacodynamics relates the concentration of an anti-infective agent to its effect, specifically to its antimicrobial effect. Concentration-dependent effects The minimum inhibitory concentration (MIC) and minimum bactericidal concentration are used to measure ''in vitro'' activity of antimicrobial agents. They are good indicators of antimicrobial potency, but don't give any information relating to time-dependent antimicrobial killing (the so-called post antibiotic effect). Post-antibiotic effect The post-antibiotic effect (PAE) is defined as persistent suppression of bacterial growth after a brief exposure (1 or 2 hours) of bacteria to an antibiotic ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Antimicrobial Agents And Chemotherapy
''Antimicrobial Agents and Chemotherapy'' is a peer-reviewed scientific journal published by the American Society for Microbiology. It covers antimicrobial, antiviral, antifungal, and antiparasitic agents and chemotherapy. The editor-in-chief is Cesar A. Arias (University of Texas Health Science Center at Houston). It was established in 1972 by Gladys Lounsbury Hobby. __TOC__ Abstracting and indexing The journal is abstracted and indexed in: According to the ''Journal Citation Reports'', its 2021 impact factor The impact factor (IF) or journal impact factor (JIF) of an academic journal is a scientometric index calculated by Clarivate that reflects the yearly mean number of citations of articles published in the last two years in a given journal, as ... is 5.938, ranking it 51st out of 279 journals in the category ''Pharmacology & Pharmacy'' and 35th out of 137 journals in the category ''Microbiology''. References External links * Delayed open access journals Pub ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Diazomethane
Diazomethane is the chemical compound CH2N2, discovered by German chemist Hans von Pechmann in 1894. It is the simplest diazo compound. In the pure form at room temperature, it is an extremely sensitive explosive yellow gas; thus, it is almost universally used as a solution in diethyl ether. The compound is a popular methylating agent in the laboratory, but it is too hazardous to be employed on an industrial scale without special precautions. Use of diazomethane has been significantly reduced by the introduction of the safer and equivalent reagent trimethylsilyldiazomethane. Use For safety and convenience diazomethane is always prepared as needed as a solution in ether and used as such. It converts carboxylic acids to methyl esters and phenols into their methyl ethers. The reaction is thought to proceed via proton transfer from carboxylic acid to diazomethane to give methyldiazonium cation, which reacts with the carboxylate ion to give the methyl ester and nitrogen gas. ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Ozonolysis
In organic chemistry, ozonolysis is an organic reaction where the unsaturated bonds of alkenes (), alkynes (), or azo compounds () are cleaved with ozone (). Alkenes and alkynes form organic compounds in which the multiple carbon–carbon bond has been replaced by a carbonyl () group while azo compounds form nitrosamines (). The outcome of the reaction depends on the type of multiple bond being oxidized and the work-up conditions. Ozonolysis of alkenes Alkenes can be oxidized with ozone to form alcohols, aldehydes or ketones, or carboxylic acids. In a typical procedure, ozone is bubbled through a solution of the alkene in methanol at −78 °C until the solution takes on a characteristic blue color, which is due to unreacted ozone. This indicates complete consumption of the alkene. Alternatively, various other chemicals can be used as indicators of this endpoint by detecting the presence of ozone. If ozonolysis is performed by bubbling a stream of ozone-enriched oxyg ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
1,8-Diazabicycloundec-7-ene
1,8-Diazabicyclo .4.0ndec-7-ene, or more commonly DBU, is a chemical compound and belongs to the class of amidine compounds. It is used in organic synthesis as a catalyst, a complexing ligand, and a non-nucleophilic base. Occurrence Although all commercially available DBU is produced synthetically, it may also be isolated from the sea sponge '' Niphates digitalis''. The biosynthesis of DBU has been proposed to begin with 1,6-hexanedial and 1,3-diaminopropane. Uses As a reagent in organic chemistry, DBU is used as a catalyst, a complexing ligand, and a non-nucleophilic base. It is also used as a curing agent for epoxy resins. It is used in the separation of fullerenes in conjunction with trimethylbenzene. It reacts with C70 and higher fullerenes, but not with to C60 It is also used as a catalyst in the production of polyurethanes. It also exhibited its dual character (base and nucleophile) in the synthesis of aryl- and styryl-terminal acetylenes. See also * 1,5-Diazabicy ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Trifluoroacetic Acid
Trifluoroacetic acid (TFA) is an organofluorine compound with the chemical formula CF3CO2H. It is a structural analogue of acetic acid with all three of the acetyl group's hydrogen atoms replaced by fluorine atoms and is a colorless liquid with a vinegar-like odor. TFA is a stronger acid than acetic acid, having an acid ionisation constant, ''K''a, that is approximately 34,000 times higher, as the highly electronegative fluorine atoms and consequent electron-withdrawing nature of the trifluoromethyl group weakens the oxygen-hydrogen bond (allowing for greater acidity) and stabilises the anionic conjugate base. TFA is widely used in organic chemistry for various purposes. Synthesis TFA is prepared industrially by the electrofluorination of acetyl chloride or acetic anhydride, followed by hydrolysis of the resulting trifluoroacetyl fluoride: : + 4 → + 3 + : + → + Where desired, this compound may be dried by addition of trifluoroacetic anhydride. An older route ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |