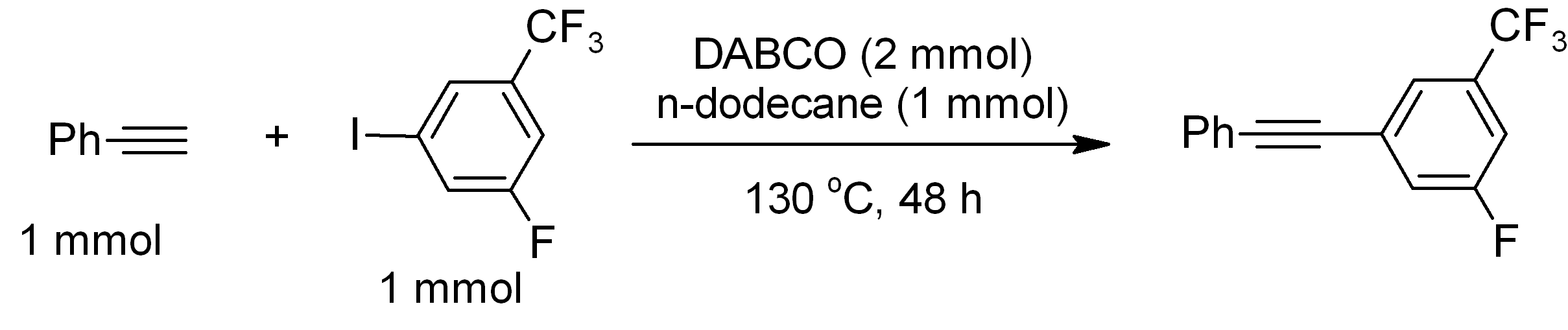
|
1,8-Diazabicycloundec-7-ene
1,8-Diazabicyclo .4.0ndec-7-ene, or more commonly DBU, is a chemical compound and belongs to the class of amidine compounds. It is used in organic synthesis as a catalyst, a complexing ligand, and a non-nucleophilic base. Occurrence Although all commercially available DBU is produced synthetically, it may also be isolated from the sea sponge '' Niphates digitalis''. The biosynthesis of DBU has been proposed to begin with 1,6-hexanedial and 1,3-diaminopropane. Uses As a reagent in organic chemistry, DBU is used as a catalyst, a complexing ligand, and a non-nucleophilic base. It is also used as a curing agent for epoxy resins. It is used in the separation of fullerenes in conjunction with trimethylbenzene. It reacts with C70 and higher fullerenes, but not with to C60 It is also used as a catalyst in the production of polyurethanes. It also exhibited its dual character (base and nucleophile) in the synthesis of aryl- and styryl-terminal acetylenes. See also * 1,5-Diazabic ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Amidines
Amidines are organic compounds with the functional group RC(NR)NR2, where the R groups can be the same or different. They are the imine derivatives of amides (RC(O)NR2). The simplest amidine is formamidine, HC(=NH)NH2. Examples of amidines include: * DBU * diminazene * benzamidine * Pentamidine * Paranyline Preparation A common route to primary amidines is the Pinner reaction. Reaction of the nitrile with alcohol in the presence of acid gives an iminoether. Treatment of the resulting compound with ammonia then completes the conversion to the amidine. Instead of using a Bronsted acid, Lewis acids such as aluminium trichloride promote the direct amination of nitriles. They are also generated by amination of an imidoyl chloride. They are also prepared by the addition of organolithium reagents to diimines, followed by protonation or alkylation. Dimethylformamide acetal reacts with primary amines to give amidines: :Me2NC(H)(OMe)2 + RNH2 → Me2NC=NHR + 2 MeOH Properties and appl ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Non-nucleophilic Base
As the name suggests, a non-nucleophilic base is a sterically hindered organic base that is a poor nucleophile. Normal bases are also nucleophiles, but often chemists seek the proton-removing ability of a base without any other functions. Typical non-nucleophilic bases are bulky, such that protons can attach to the basic center but alkylation and complexation is inhibited. Non-nucleophilic bases A variety of amines and nitrogen heterocycles are useful bases of moderate strength (pKa of conjugate acid * ''N'',''N''-Diisopropylethylamine (DIPEA, also called Hünig's Base), p * 1,8-Diazabicycloundec-7-ene (DBU) - useful for E2 elimination reactions, pKa = 13.5 * 1,5-Diazabicyclo(4.3.0)non-5-ene (DBN) - comparable to DBU * 2,6-Di-tert-butylpyridine, a weak non-nucleophilic base pKa = 3.58 * Phosphazene bases, such as t-Bu-P4''Activation in anionic polymerization: Why phosphazene bases are very exciting promoters'' S. Boileau, N. Illy Prog. Polym. Sci., 2011, 36, 1132-1151, {{doi, 10.1 ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Niphates Digitalis
''Niphates digitalis'', commonly known as the pink vase sponge, is a species of sea sponge belonging to the family Niphatidae. It is native to the Florida Keys, The Bahamas, and the Caribbean including the Netherlands Antilles. The species was first described by Jean-Baptiste Lamarck in 1814. Characteristics The pink vase sponge is a demosponge that can grow up to 50 cm in height and width, but is more commonly smaller. It is normally vase-, tube-, or cup-shaped with a narrow base and broader top, and somewhat flattened when viewed in cross section. Rarely, it can grow as a fan shape. Despite its name, the colour has been observed as blue, gray, and lavender, as well as "purplish to pink". The surface is coarse and porous with 6-mm-long conules or spines. Chemistry Compounds extracted from the pink vase sponge have been investigated for their possible use in the treatment of castration recurrent prostate cancer. A common reagent used in organic chemistry 1,8-diazabic ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Amidine
Amidines are organic compounds with the functional group RC(NR)NR2, where the R groups can be the same or different. They are the imine derivatives of amides (RC(O)NR2). The simplest amidine is formamidine, HC(=NH)NH2. Examples of amidines include: * DBU * diminazene * benzamidine * Pentamidine * Paranyline Preparation A common route to primary amidines is the Pinner reaction. Reaction of the nitrile with alcohol in the presence of acid gives an iminoether. Treatment of the resulting compound with ammonia then completes the conversion to the amidine. Instead of using a Bronsted acid, Lewis acids such as aluminium trichloride promote the direct amination of nitriles. They are also generated by amination of an imidoyl chloride. They are also prepared by the addition of organolithium reagents to diimines, followed by protonation or alkylation. Dimethylformamide acetal reacts with primary amines to give amidines: :Me2NC(H)(OMe)2 + RNH2 → Me2NC=NHR + 2 MeOH Properties and appl ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Chemical Compound
A chemical compound is a chemical substance composed of many identical molecules (or molecular entities) containing atoms from more than one chemical element held together by chemical bonds. A molecule consisting of atoms of only one element is therefore not a compound. A compound can be transformed into a different substance by a chemical reaction, which may involve interactions with other substances. In this process, bonds between atoms may be broken and/or new bonds formed. There are four major types of compounds, distinguished by how the constituent atoms are bonded together. Molecular compounds are held together by covalent bonds; ionic compounds are held together by ionic bonds; intermetallic compounds are held together by metallic bonds; coordination complexes are held together by coordinate covalent bonds. Non-stoichiometric compounds form a disputed marginal case. A chemical formula specifies the number of atoms of each element in a compound molecule, using ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Fullerene
A fullerene is an allotrope of carbon whose molecule consists of carbon atoms connected by single and double bonds so as to form a closed or partially closed mesh, with fused rings of five to seven atoms. The molecule may be a hollow sphere, ellipsoid, tube, or many other shapes and sizes. Graphene (isolated atomic layers of graphite), which is a flat mesh of regular hexagonal rings, can be seen as an extreme member of the family. Fullerenes with a closed mesh topology are informally denoted by their empirical formula C''n'', often written C''n'', where ''n'' is the number of carbon atoms. However, for some values of ''n'' there may be more than one isomer. The family is named after buckminsterfullerene (C60), the most famous member, which in turn is named after Buckminster Fuller. The closed fullerenes, especially C60, are also informally called buckyballs for their resemblance to the standard ball of association football ("soccer"). Nested closed fullerenes have been na ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
DABCO
DABCO (1,4-diazabicyclo .2.2ctane), also known as triethylenediamine or TEDA, is a bicyclic organic compound with the formula N2(C2H4)3. This colorless solid is a highly nucleophilic tertiary amine base, which is used as a catalyst and reagent in polymerization and organic synthesis. It is similar in structure to quinuclidine, but the latter has one of the nitrogen atoms replaced by a carbon atom. Reactions The p''K''a of DABCOsup>+ (the protonated derivative) is 8.8, which is almost the same as ordinary alkylamines. The nucleophilicity of the amine is high because the amine centers are unhindered. It is sufficiently basic to promote C–C coupling of terminal acetylenes, for example, phenylacetylene couples with electron-deficient iodoarenes. : Catalyst DABCO is used as a base-catalyst for: *formation of polyurethane from alcohol and isocyanate functionalized monomers and pre-polymers. * Baylis-Hillman reactions of aldehydes and unsaturated ketones and aldehydes. : Lewi ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Polyurethane
Polyurethane (; often abbreviated PUR and PU) refers to a class of polymers composed of organic units joined by carbamate (urethane) links. In contrast to other common polymers such as polyethylene and polystyrene, polyurethane is produced from a wide range of starting materials. This chemical variety produces polyurethanes with different chemical structures leading to many different applications. These include rigid and flexible foams, varnishes and coatings, adhesives, electrical potting compounds, and fibers such as spandex and PUL. Foams are the largest application accounting for 67% of all polyurethane produced in 2016. A polyurethane is typically produced by reacting an isocyanate with a polyol. Since a polyurethane contains two types of monomers, which polymerize one after the other, they are classed as alternating copolymers. Both the isocyanates and polyols used to make a polyurethane contain two or more functional groups per molecule. Global production in 2019 wa ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
1,2,4-Trimethylbenzene
1,2,4-Trimethylbenzene, also known as pseudocumene, is an organic compound with the chemical formula CH(CH). Classified as an aromatic hydrocarbon, it is a flammable colorless liquid with a strong odor. It is nearly insoluble in water but soluble in organic solvents. It occurs naturally in coal tar and petroleum (about 3%). It is one of the three isomers of trimethylbenzene. Production Industrially, it is isolated from the C aromatic hydrocarbon fraction during petroleum distillation. Approximately 40% of this fraction is 1,2,4-trimethylbenzene. It is also generated by methylation of toluene and xylenes and the disproportionation of xylene over aluminosilicate catalysts.Karl Griesbaum, Arno Behr, Dieter Biedenkapp, Heinz-Werner Voges, Dorothea Garbe, Christian Paetz, Gerd Collin, Dieter Mayer, Hartmut Höke "Hydrocarbons" in Ullmann's Encyclopedia of Industrial Chemistry 2002 Wiley-VCH, Weinheim. Uses Pseudocumene is a precursor to mellitic anhydride, from which high performanc ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Reagent
In chemistry, a reagent ( ) or analytical reagent is a substance or compound added to a system to cause a chemical reaction, or test if one occurs. The terms ''reactant'' and ''reagent'' are often used interchangeably, but reactant specifies a substance ''consumed'' in the course of a chemical reaction. ''Solvents'', though involved in the reaction mechanism, are usually not called reactants. Similarly, '' catalysts'' are not consumed by the reaction, so they are not reactants. In biochemistry, especially in connection with enzyme-catalyzed reactions, the reactants are commonly called substrates. Definitions Organic chemistry In organic chemistry, the term "reagent" denotes a chemical ingredient (a compound or mixture, typically of inorganic or small organic molecules) introduced to cause the desired transformation of an organic substance. Examples include the Collins reagent, Fenton's reagent, and Grignard reagents. Analytical chemistry In analytical chemistry, a reag ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Epoxy
Epoxy is the family of basic components or cured end products of epoxy resins. Epoxy resins, also known as polyepoxides, are a class of reactive prepolymers and polymers which contain epoxide groups. The epoxide functional group is also collectively called ''epoxy''. The IUPAC name for an epoxide group is an oxirane. Epoxy resins may be reacted ( cross-linked) either with themselves through catalytic homopolymerisation, or with a wide range of co-reactants including polyfunctional amines, acids (and acid anhydrides), phenols, alcohols and thiols (usually called mercaptans). These co-reactants are often referred to as hardeners or curatives, and the cross-linking reaction is commonly referred to as curing. Reaction of polyepoxides with themselves or with polyfunctional hardeners forms a thermosetting polymer, often with favorable mechanical properties and high thermal and chemical resistance. Epoxy has a wide range of applications, including metal coatings, composites, ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |