|
Cd(CN)2
Cadmium cyanide is an inorganic compound with the formula Cd(CN)2. It is a white crystalline compound that is used in electroplating. It is very toxic, along with other cadmium and cyanide compounds. Preparation and structure Cadmium cyanide is prepared commercially by treating cadmium hydroxide with hydrogen cyanide:Karl-Heinz Schulte-Schrepping, Magnus Piscator "Cadmium and Cadmium Compounds" in Ullmann's Encyclopedia of Industrial Chemistry, 2007 Wiley-VCH, Weinheim. . : Cd(OH)2 + 2 HCN → Cd(CN)2 + 2 H2O It can also be generated from tetracyanocadmate: : d(CN)4sup>2− + CdCl2 → 2 Cd(CN)2 + 2 Cl− Cadmium cyanide and zinc cyanide adopt similar structures. As such, each metal has tetrahedral coordination sphere. Cyanide ligands interconnect pairs of metal centers. Two of the resulting diamondoid structures are interpenetrated. The structure is related to that of cristobalite, a polymorphs of SiO2. This structural similarity of cadmium dicyanide and cristobal ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Zinc Cyanide
Zinc cyanide is the inorganic compound with the formula Zinc, Zn(Cyanide, CN)2. It is a white solid that is used mainly for electroplating zinc but also has more specialized applications for the organic synthesis, synthesis of organic compounds. Structure In Zn(CN)2, zinc adopts the tetrahedral coordination environment, all linked by bridging ligand, bridging cyanide ligands. The structure consists of two "interpenetrating" structures (blue and red in the picture above). Such motifs are sometimes called "expanded diamondoid" structures. Some forms of SiO2 adopt a similar structure, wherein the tetrahedral Si centres are linked by oxides. The cyanide group shows head to tail disorder with any zinc atom having between one and four carbon neighbours, and the remaining being nitrogen atoms. It shows one of the largest Negative thermal expansion, negative coefficients of thermal expansion (exceeding the previous record holder, zirconium tungstate). Chemical properties Typical for an ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Clathrate
A clathrate is a chemical substance consisting of a lattice that traps or contains molecules. The word ''clathrate'' is derived from the Latin (), meaning ‘with bars, latticed’. Most clathrate compounds are polymeric and completely envelop the guest molecule, but in modern usage clathrates also include host–guest complexes and inclusion compounds.Atwood, J. L. (2012) "Inclusion Compounds" in ''Ullmann's Encyclopedia of Industrial Chemistry''. Wiley-VCH, Weinheim. According to IUPAC, clathrates are inclusion compounds "in which the guest molecule is in a cage formed by the host molecule or by a lattice of host molecules." The term refers to many molecular hosts, including calixarenes and cyclodextrins and even some inorganic polymers such as zeolites. Clathrates can be divided into two categories: clathrate hydrates and inorganic clathrates. Each clathrate is made up of a framework and guests that reside the framework. Most common clathrate crystal structures can be co ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Zinc Cyanide
Zinc cyanide is the inorganic compound with the formula Zinc, Zn(Cyanide, CN)2. It is a white solid that is used mainly for electroplating zinc but also has more specialized applications for the organic synthesis, synthesis of organic compounds. Structure In Zn(CN)2, zinc adopts the tetrahedral coordination environment, all linked by bridging ligand, bridging cyanide ligands. The structure consists of two "interpenetrating" structures (blue and red in the picture above). Such motifs are sometimes called "expanded diamondoid" structures. Some forms of SiO2 adopt a similar structure, wherein the tetrahedral Si centres are linked by oxides. The cyanide group shows head to tail disorder with any zinc atom having between one and four carbon neighbours, and the remaining being nitrogen atoms. It shows one of the largest Negative thermal expansion, negative coefficients of thermal expansion (exceeding the previous record holder, zirconium tungstate). Chemical properties Typical for an ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Interpenetrating Polymer Network
An Interpenetrating polymer network (IPN) is a polymer comprising two or more networks which are at least partially interlaced on a polymer scale but not covalently bonded to each other. The network cannot be separated unless chemical bonds are broken. The two or more networks can be envisioned to be entangled in such a way that they are concatenated and cannot be pulled apart, but not bonded to each other by any chemical bond. Simply mixing two or more polymers does not create an interpenetrating polymer network (polymer blend), nor does creating a polymer network out of more than one kind of monomers which are bonded to each other to form one network (heteropolymer or copolymer In polymer chemistry, a copolymer is a polymer derived from more than one species of monomer. The polymerization of monomers into copolymers is called copolymerization. Copolymers obtained from the copolymerization of two monomer species are some ...). There are semi-interpenetrating polymer networks ( ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Ethanol
Ethanol (abbr. EtOH; also called ethyl alcohol, grain alcohol, drinking alcohol, or simply alcohol) is an organic compound. It is an alcohol with the chemical formula . Its formula can be also written as or (an ethyl group linked to a hydroxyl group). Ethanol is a volatile, flammable, colorless liquid with a characteristic wine-like odor and pungent taste. It is a psychoactive recreational drug, the active ingredient in alcoholic drinks. Ethanol is naturally produced by the fermentation process of sugars by yeasts or via petrochemical processes such as ethylene hydration. It has medical applications as an antiseptic and disinfectant. It is used as a chemical solvent and in the synthesis of organic compounds, and as a fuel source. Ethanol also can be dehydrated to make ethylene, an important chemical feedstock. As of 2006, world production of ethanol was , coming mostly from Brazil and the U.S. Etymology ''Ethanol'' is the systematic name defined by the Interna ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Corrosion
Corrosion is a natural process that converts a refined metal into a more chemically stable oxide. It is the gradual deterioration of materials (usually a metal) by chemical or electrochemical reaction with their environment. Corrosion engineering is the field dedicated to controlling and preventing corrosion. In the most common use of the word, this means electrochemical oxidation of metal in reaction with an oxidant such as oxygen, hydrogen or hydroxide. Rusting, the formation of iron oxides, is a well-known example of electrochemical corrosion. This type of damage typically produces oxide(s) or salt(s) of the original metal and results in a distinctive orange colouration. Corrosion can also occur in materials other than metals, such as ceramics or polymers, although in this context, the term "degradation" is more common. Corrosion degrades the useful properties of materials and structures including strength, appearance and permeability to liquids and gases. Many ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Electrolyte
An electrolyte is a medium containing ions that is electrically conducting through the movement of those ions, but not conducting electrons. This includes most soluble salts, acids, and bases dissolved in a polar solvent, such as water. Upon dissolving, the substance separates into cations and anions, which disperse uniformly throughout the solvent. Solid-state electrolytes also exist. In medicine and sometimes in chemistry, the term electrolyte refers to the substance that is dissolved. Electrically, such a solution is neutral. If an electric potential is applied to such a solution, the cations of the solution are drawn to the electrode that has an abundance of electrons, while the anions are drawn to the electrode that has a deficit of electrons. The movement of anions and cations in opposite directions within the solution amounts to a current. Some gases, such as hydrogen chloride (HCl), under conditions of high temperature or low pressure can also function as elect ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Polymorphism (materials Science)
In materials science, polymorphism describes the existence of a solid material in more than one form or crystal structure. Polymorphism is a form of isomerism. Any crystalline material can exhibit the phenomenon. Allotropy refers to polymorphism for chemical elements. Polymorphism is of practical relevance to pharmaceuticals, agrochemicals, pigments, dyestuffs, foods, and explosives. According to IUPAC, a polymorphic transition is "A reversible transition of a solid crystalline phase at a certain temperature and pressure (the inversion point) to another phase of the same chemical composition with a different crystal structure." According to McCrone, polymorphs are "different in crystal structure but identical in the liquid or vapor states." Materials with two polymorphs are called dimorphic, with three polymorphs, trimorphic, etc. Examples Many compounds exhibit polymorphism. It has been claimed that "every compound has different polymorphic forms, and that, in general, the nu ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Cristobalite
Cristobalite is a mineral polymorph of silica that is formed at very high temperatures. It has the same chemical formula as quartz, SiO2, but a distinct crystal structure. Both quartz and cristobalite are polymorphs with all the members of the quartz group, which also include coesite, tridymite and stishovite. It is named after Cerro San Cristóbal in Pachuca Municipality, Hidalgo, Mexico. It is used in dentistry as a component of alginate impression materials as well as for making models of teeth. Properties Metastability Cristobalite is stable only above 1470 °C, but can crystallize and persist metastably at lower temperatures. The persistence of cristobalite outside its thermodynamic stability range occurs because the transition from cristobalite to quartz or tridymite is "reconstructive", requiring the breaking up and reforming of the silica framework. These frameworks are composed of Si O4 tetrahedra in which every oxygen atom is shared with a neighbouring ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Diamondoid
In chemistry, diamondoids are variants of the carbon cage molecule known as adamantane (C10H16), the smallest unit cage structure of the diamond crystal lattice. Diamondoids also known as nanodiamonds or condensed adamantanes may include one or more cages (adamantane, diamantane, triamantane, and higher polymantanes) as well as numerous isomeric and structural variants of adamantanes and polymantanes. These diamondoids occur naturally in petroleum deposits and have been extracted and purified into large pure crystals of polymantane molecules having more than a dozen adamantane cages per molecule. These species are of interest as molecular approximations of the diamond cubic framework, terminated with C−H bonds. Cyclohexamantane may be thought of as a nanometer-sized diamond of approximately . Examples Examples include: * Adamantane (C10H16) * Iceane (C12H18) * BC-8 (C14H20) * Diamantane (C14H20) also ''diadamantane'', two face-fused cages * Triamantane (C18H24), also ''tr ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
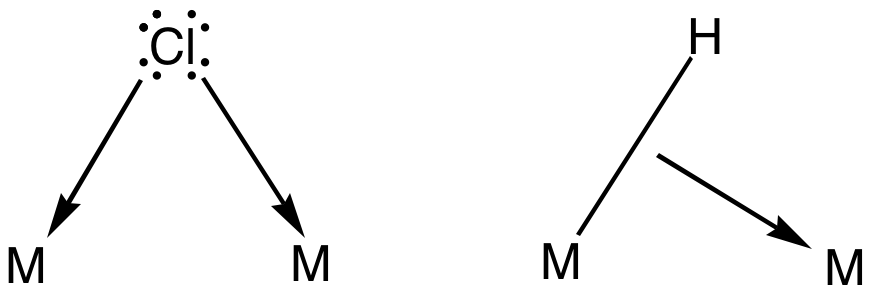
Bridging Ligand
In coordination chemistry, a bridging ligand is a ligand that connects two or more atoms, usually metal ions. The ligand may be atomic or polyatomic. Virtually all complex organic compounds can serve as bridging ligands, so the term is usually restricted to small ligands such as pseudohalides or to ligands that are specifically designed to link two metals. In naming a complex wherein a single atom bridges two metals, the bridging ligand is preceded by the Greek letter mu, μ, with a subscript number denoting the number of metals bound to the bridging ligand. μ2 is often denoted simply as μ. When describing coordination complexes care should be taken not to confuse μ with η ('eta'), which relates to hapticity. Ligands that are not bridging are called terminal ligands. List of bridging ligands Virtually all ligands are known to bridge, with the exception of amines and ammonia. Common bridging ligands include most of the common anions. Many simple organic ligands form ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Tetrahedral Molecular Geometry
In a tetrahedral molecular geometry, a central atom is located at the center with four substituents that are located at the corners of a tetrahedron. The bond angles are cos−1(−) = 109.4712206...° ≈ 109.5° when all four substituents are the same, as in methane () as well as its heavier analogues. Methane and other perfectly symmetrical tetrahedral molecules belong to point group Td, but most tetrahedral molecules have lower symmetry. Tetrahedral molecules can be chiral. Tetrahedral bond angle The bond angle for a symmetric tetrahedral molecule such as CH4 may be calculated using the dot product of two vectors. As shown in the diagram, the molecule can be inscribed in a cube with the tetravalent atom (e.g. carbon) at the cube centre which is the origin of coordinates, O. The four monovalent atoms (e.g. hydrogens) are at four corners of the cube (A, B, C, D) chosen so that no two atoms are at adjacent corners linked by only one cube edge. If the edge length of the cube ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
2CCl4.jpg)
2.jpg)