|
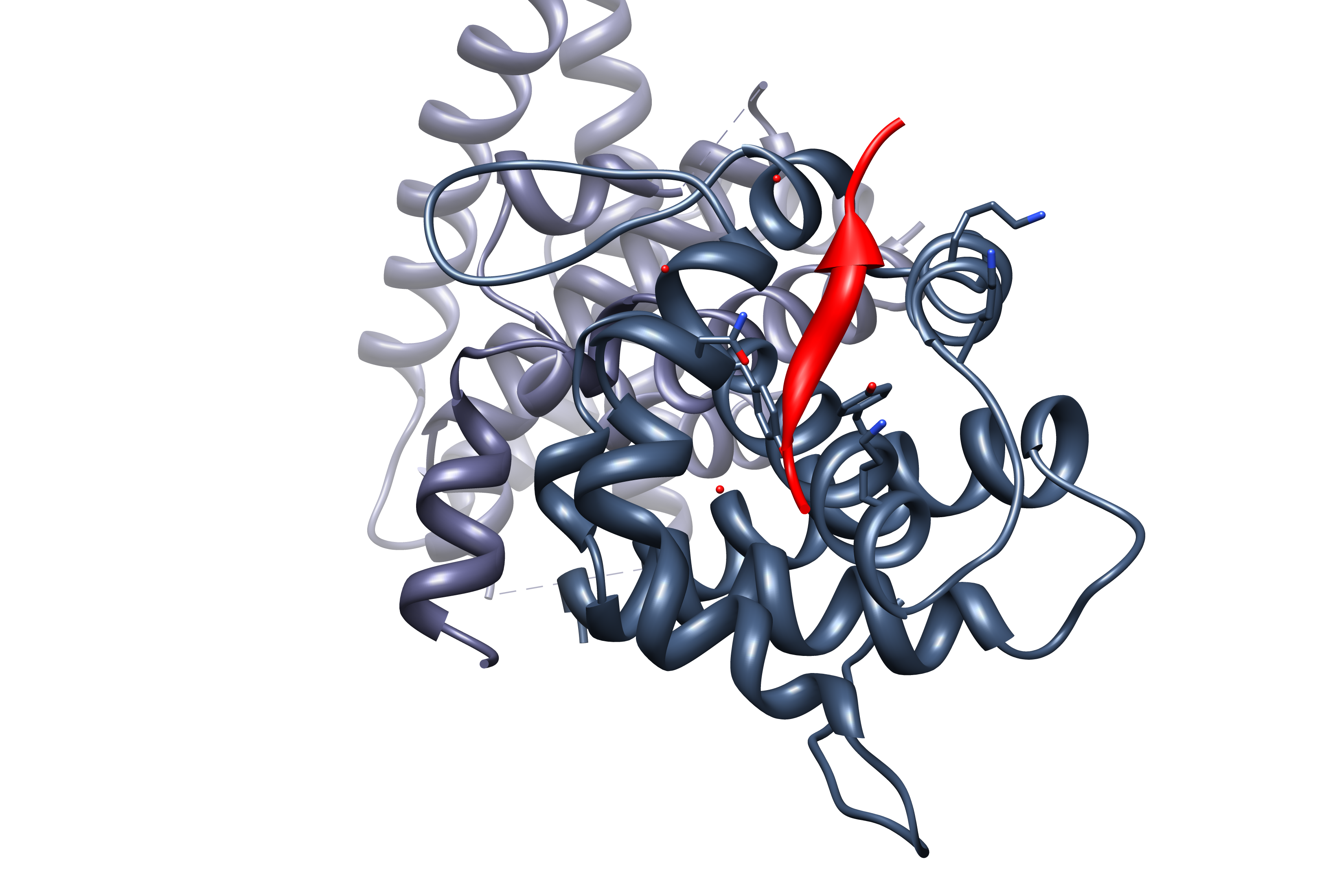
Caspase Recruitment Domain
Caspase recruitment domains, or caspase activation and recruitment domains (CARDs), are interaction motifs found in a wide array of proteins, typically those involved in processes relating to inflammation and apoptosis. These domains mediate the formation of larger protein complexes via direct interactions between individual CARDs. CARD domains are found on a strikingly wide range of proteins, including helicases, kinases, mitochondrial proteins, caspases, and other cytoplasmic factors. Basic features CARD domains are a subclass of protein motif known as the death fold, which features an arrangement of six to seven antiparallel alpha helices with a hydrophobic core and an outer face composed of charged residues. Other motifs in this class include the pyrin domain (PYD), death domain (DD), and death effector domain (DED), all of which also function primarily in regulation of apoptosis and inflammatory responses. In apoptosis CARD domains were originally characterized based on ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Short Linear Motif
In molecular biology short linear motifs (SLiMs), linear motifs or minimotifs are short stretches of protein sequence that mediate protein–protein interaction. The first definition was given by Tim Hunt: "The sequences of many proteins contain short, conserved motifs that are involved in recognition and targeting activities, often separate from other functional properties of the molecule in which they occur. These motifs are linear, in the sense that three-dimensional organization is not required to bring distant segments of the molecule together to make the recognizable unit. The conservation of these motifs varies: some are highly conserved while others, for example, allow substitutions that retain only a certain pattern of charge across the motif." Attributes SLiMs are generally situated in intrinsically disordered regions (over 80% of known SLiMs), however, upon interaction with a structured partner secondary structure is often induced. The majority of annotated SLiMs con ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
NF-κB
Nuclear factor kappa-light-chain-enhancer of activated B cells (NF-κB) is a protein complex that controls transcription of DNA, cytokine production and cell survival. NF-κB is found in almost all animal cell types and is involved in cellular responses to stimuli such as stress, cytokines, free radicals, heavy metals, ultraviolet irradiation, oxidized LDL, and bacterial or viral antigens. NF-κB plays a key role in regulating the immune response to infection. Incorrect regulation of NF-κB has been linked to cancer, inflammatory and autoimmune diseases, septic shock, viral infection, and improper immune development. NF-κB has also been implicated in processes of synaptic plasticity and memory. Discovery NF-κB was discovered by Ranjan Sen in the lab of Nobel laureate David Baltimore via its interaction with an 11-base pair sequence in the immunoglobulin light-chain enhancer in B cells. Later work by Alexander Poltorak and Bruno Lemaitre in mice and ''Drosophila'' frui ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
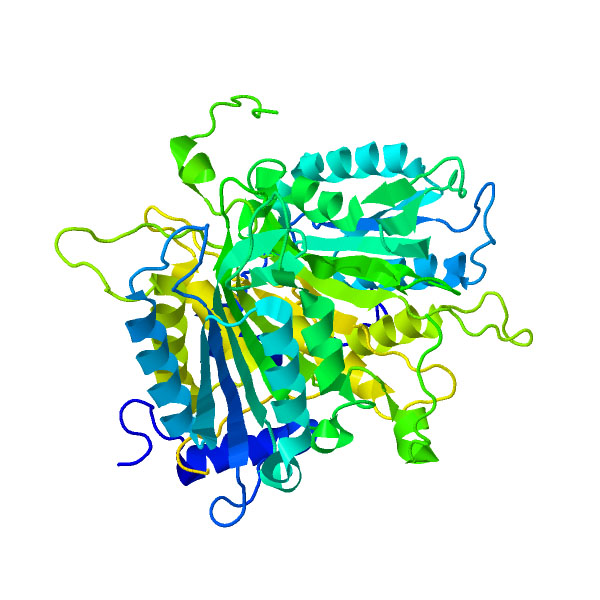
Caspase 4
Caspase 4 is an enzyme that proteolytically cleaves other proteins at an aspartic acid residue (LEVD-), and belongs to a family of cysteine proteases called caspases. The function of caspase 4 is not fully known, but it is believed to be an inflammatory caspase, along with caspase 1, caspase 5 (and the murine homolog caspase 11), with a role in the immune system The immune system is a network of biological processes that protects an organism from diseases. It detects and responds to a wide variety of pathogens, from viruses to parasitic worms, as well as cancer cells and objects such as wood splint .... The Nonsteroidal anti-inflammatory drug, anti-inflammatory drug indoprofen is an inhibitor of the activity of the caspase-4 enzyme. See also * The Proteolysis Map * Caspase References External links * The MEROPS online database for peptidases and their inhibitorsC14.007 EC 3.4.22 Caspases {{gene-11-stub ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Caspase 2
Caspase 2 also known as CASP2 is an enzyme that, in humans, is encoded by the ''CASP2'' gene. ''CASP2'' orthologs have been identified in nearly all mammals for which complete genome data are available. Unique orthologs are also present in birds, lizards, lissamphibians, and teleosts. Function Sequential activation of caspases plays a central role in the execution-phase of cell apoptosis. Caspases exist as inactive proenzymes that undergo proteolytic processing at conserved aspartic residues to produce two subunits, large and small, that dimerize to form the active enzyme. The proteolytic cleavage of this protein is induced by a variety of apoptotic stimuli. Caspase 2 proteolytically cleaves other proteins. It belongs to a family of cysteine proteases called caspases that cleave proteins only at an amino acid following an aspartic acid residue. Within this family, caspase 2 is part of the Ich-1 subfamily. It is one of the most conserved caspases in different species of anim ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Caspase 1
Caspase-1/Interleukin-1 converting enzyme (ICE) is an evolutionarily conserved enzyme that proteolytically cleaves other proteins, such as the precursors of the inflammatory cytokines interleukin 1β and interleukin 18 as well as the pyroptosis inducer Gasdermin D, into active mature peptides. It plays a central role in cell immunity as an inflammatory response initiator. Once activated through formation of an inflammasome complex, it initiates a proinflammatory response through the cleavage and thus activation of the two inflammatory cytokines, interleukin 1β (IL-1β) and interleukin 18 (IL-18) as well as pyroptosis, a programmed lytic cell death pathway, through cleavage of Gasdermin D. The two inflammatory cytokines activated by Caspase-1 are excreted from the cell to further induce the inflammatory response in neighboring cells. Cellular expression Caspase-1 is evolutionarily conserved in many eukaryotes of the Kingdom Animalia. Due to its role in the inflammatory immune ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
BIRC3
Baculoviral IAP repeat-containing protein3 (also known as cIAP2) is a protein that in humans is encoded by the ''BIRC3'' gene. cIAP2 is a member of the inhibitor of apoptosis family that inhibit apoptosis by interfering with the activation of caspases. The encoded protein inhibits apoptosis induced by serum deprivation but does not affect apoptosis resulting from exposure to menadione, a potent inducer of free radicals. The cIAP2 protein contains three BIR domains, a UBA domain, a CARD domain and a RING finger domain. Transcript variants encoding the same isoform have been identified. Interactions Baculoviral IAP repeat-containing protein 3 has been shown to interact with: * CASP9, * RIPK1, * TRAF1, * TRAF2, and * UBE2D2 Ubiquitin-conjugating enzyme E2 D2 is a protein that in humans is encoded by the ''UBE2D2'' gene. Function The modification of proteins with ubiquitin is an important cellular mechanism for targeting abnormal or short-lived proteins for degrad .... Refe ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Inhibitor Of Apoptosis
Inhibitors of apoptosis are a group of proteins that mainly act on the intrinsic pathway that block programmed cell death, which can frequently lead to cancer or other effects for the cell if mutated or improperly regulated. Many of these inhibitors act to block caspases, a family of cysteine proteases that play an integral role in apoptosis. Some of these inhibitors include the Bcl-2 family, viral inhibitor crmA, and IAP's. Apoptosis, or programmed cell death, is a highly regulated process used by many multicellular organisms. Like any regulated process, apoptosis is subject to either activation or inhibition by a variety of chemical factors. Apoptosis can be triggered through two main pathways; extrinsic and intrinsic pathways. The extrinsic pathway mostly involves extracellular signals triggering intracellular apoptosis mechanisms by binding to receptors in the cell membrane and sending signals from the outside of the cell. Intrinsic pathways involved internal cell signaling pri ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
BIRC2
Baculoviral IAP repeat-containing protein 2 (also known as cIAP1) is a protein that in humans is encoded by the ''BIRC2'' gene. Function cIAP1 is a member of the Inhibitor of Apoptosis family that inhibit apoptosis by interfering with the activation of caspases. Interactions BIRC2 has been shown to interact with: * CASP9, * DIABLO, * GSPT1, * HSP90B1, * HTRA2, * RIPK1, * RIPK2 * TNFSF14, * TRAF1, * TRAF2, and * UBC The University of British Columbia (UBC) is a public research university with campuses near Vancouver and in Kelowna, British Columbia. Established in 1908, it is British Columbia's oldest university. The university ranks among the top three .... References Further reading * * * * * * * * * * * * * * * * * * External links * {{PDB Gallery, geneid=329 Proteins Microbiology ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Familial Mediterranean Fever
Familial Mediterranean fever (FMF) is a hereditary inflammatory disorder. FMF is an autoinflammatory disease caused by mutations in Mediterranean fever gene, which encodes a 781–amino acid protein called pyrin. While all ethnic groups are susceptible to FMF, it usually occurs in people of Mediterranean origin—including Sephardic Jews, Mizrahi Jews, Ashkenazi Jews, Assyrian People, Assyrians, Armenians, Azerbaijanis, Levantine people, Levantines, Kurds, Greeks, Turkish people, Turks and Italians. The disorder has been given various names, including familial paroxysmal polyserositis, periodic peritonitis, recurrent polyserositis, benign paroxysmal peritonitis, periodic disease or periodic fever, Reimann periodic disease or Reimann syndrome, Siegal-Cattan-Mamou disease, and Wolff periodic disease. Note that "periodic fever" can also refer to any of the periodic fever syndromes. Signs and symptoms Attacks There are seven types of attacks. Ninety percent of all patients have t ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Muckle–Wells Syndrome
Muckle–Wells syndrome (MWS) is a rare autosomal dominant disease which causes sensorineural deafness and recurrent hives, and can lead to amyloidosis. Individuals with MWS often have episodic fever, chills, and joint pain. As a result, MWS is considered a type of periodic fever syndrome. MWS is caused by a defect in the CIAS1 gene which creates the protein cryopyrin. MWS is closely related to two other syndromes, familial cold urticaria and neonatal onset multisystem inflammatory disease—in fact, all three are related to mutations in the same gene and subsumed under the term cryopyrin-associated periodic syndromes (CAPS). Sign and symptoms * Sensorineural deafness * Recurrent urticaria (hives) * Fevers * Chills * Arthralgia (painful joints) Causes MWS occurs when a mutation in the ''CIAS1'' gene, encoding for NLRP3, leads to increased activity of the protein cryopyrin. This protein is partly responsible for the body's response to damage or infection. During these states, a ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
CIAS1
NLR family pyrin domain containing 3 (NLRP3) (previously known as NACHT, LRR and PYD domains-containing protein 3 ALP3and cryopyrin), is a protein that in humans is encoded by the ''NLRP3'' gene located on the long arm of chromosome 1. NLRP3 is expressed predominantly in macrophages and as a component of the inflammasome, detects products of damaged cells such as extracellular ATP and crystalline uric acid. Activated NLRP3 in turn triggers an immune response. Mutations in the NLRP3 gene are associated with a number of organ specific autoimmune diseases. Nomenclature NACHT, LRR, and PYD are respectively acronyms for: * NACHT – NAIP (neuronal apoptosis inhibitor protein), C2TA heterokaryon_incompatibility)_and_TP1_(telomerase-associated_protein_1) *_ MHC,_HET-E_(Podospora_anserina#Heterokaryon_incompatibility">heterokaryon_incompatibility)_and_TP1_(telomerase-associated_protein_1) *_Leucine-rich_repeat">LRR_–_"leucine-rich_repeat"__and_is_synonymous_with_NLR,_for_''_o ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Cryopyrin
NLR family pyrin domain containing 3 (NLRP3) (previously known as NACHT, LRR and PYD domains-containing protein 3 ALP3and cryopyrin), is a protein that in humans is encoded by the ''NLRP3'' gene located on the long arm of chromosome 1. NLRP3 is expressed predominantly in macrophages and as a component of the inflammasome, detects products of damaged cells such as extracellular ATP and crystalline uric acid. Activated NLRP3 in turn triggers an immune response. Mutations in the NLRP3 gene are associated with a number of organ specific autoimmune diseases. Nomenclature NACHT, LRR, and PYD are respectively acronyms for: * NACHT – NAIP (neuronal apoptosis inhibitor protein), C2TA heterokaryon_incompatibility)_and_TP1_(telomerase-associated_protein_1) *_ MHC,_HET-E_(Podospora_anserina#Heterokaryon_incompatibility">heterokaryon_incompatibility)_and_TP1_(telomerase-associated_protein_1) *_Leucine-rich_repeat">LRR_–_"leucine-rich_repeat"__and_is_synonymous_with_NLR,_for_''_or_' ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |